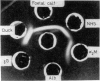
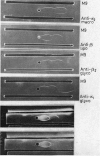
Abstract
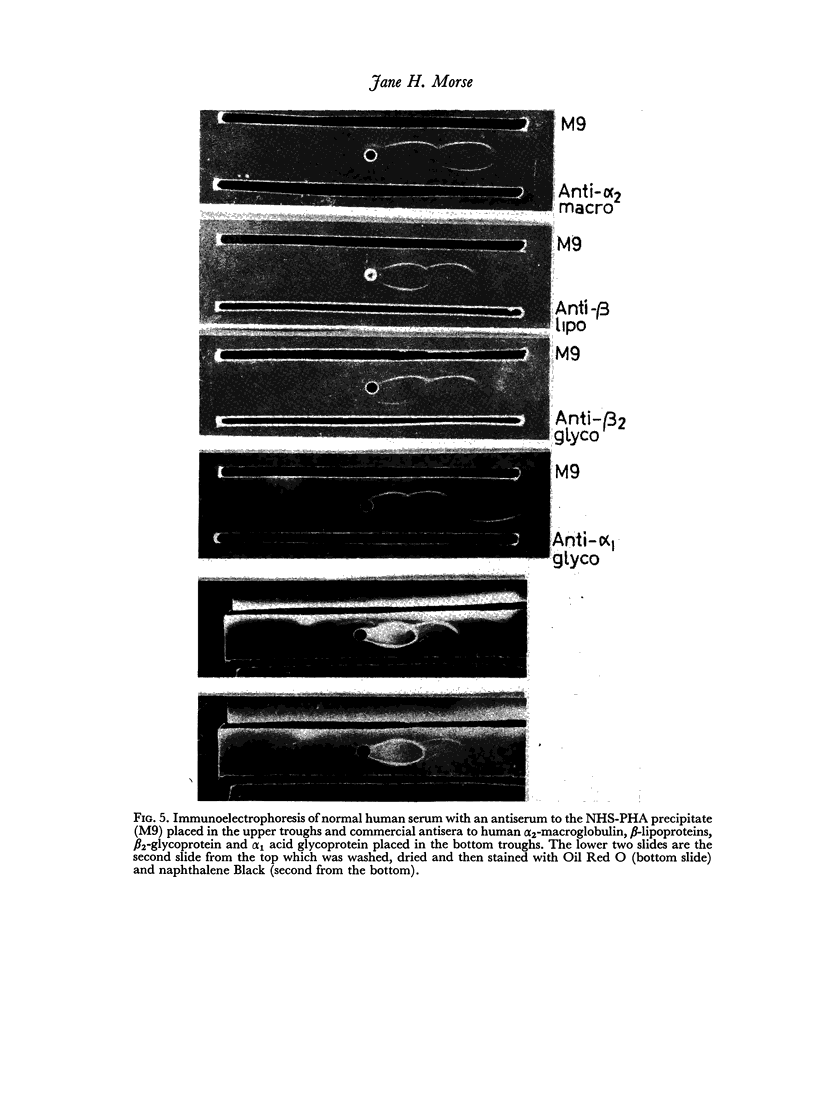
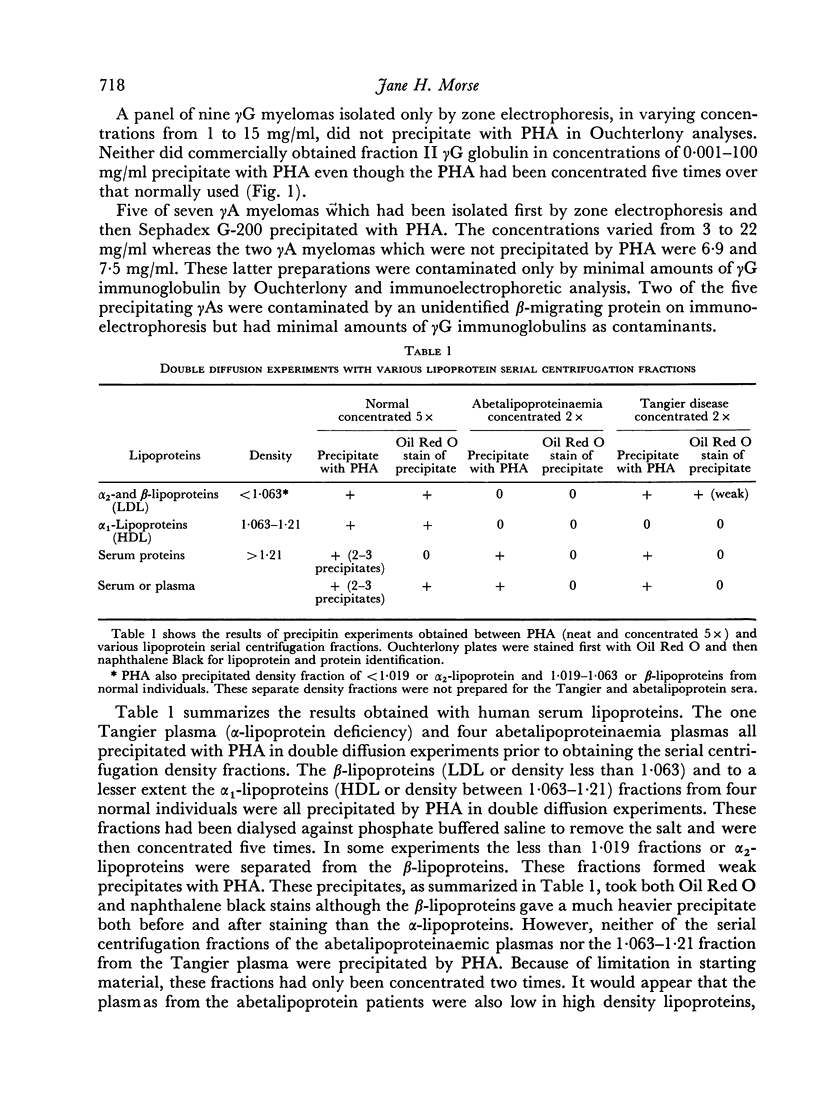
Studies of the precipitation of normal human serum by the kidney bean phytohaemagglutinin, Phaseolus vulgaris (PHA), showed that the main serum components involved were α2-macroglobulin, β-lipoproteins and γM immunoglobulins. Preparations of α1-glycoprotein, orosomucoid and some γA immunoglobulins were also precipitated by PHA. Antisera prepared against the PHA—NHS precipitate recognized α2-macroglobulin, β-lipoproteins and γM immunoglobulins, γG immunoglobulins and at least four other unidentified antigens in the β and α regions. PHA did not react with B or A, O blood group substances.
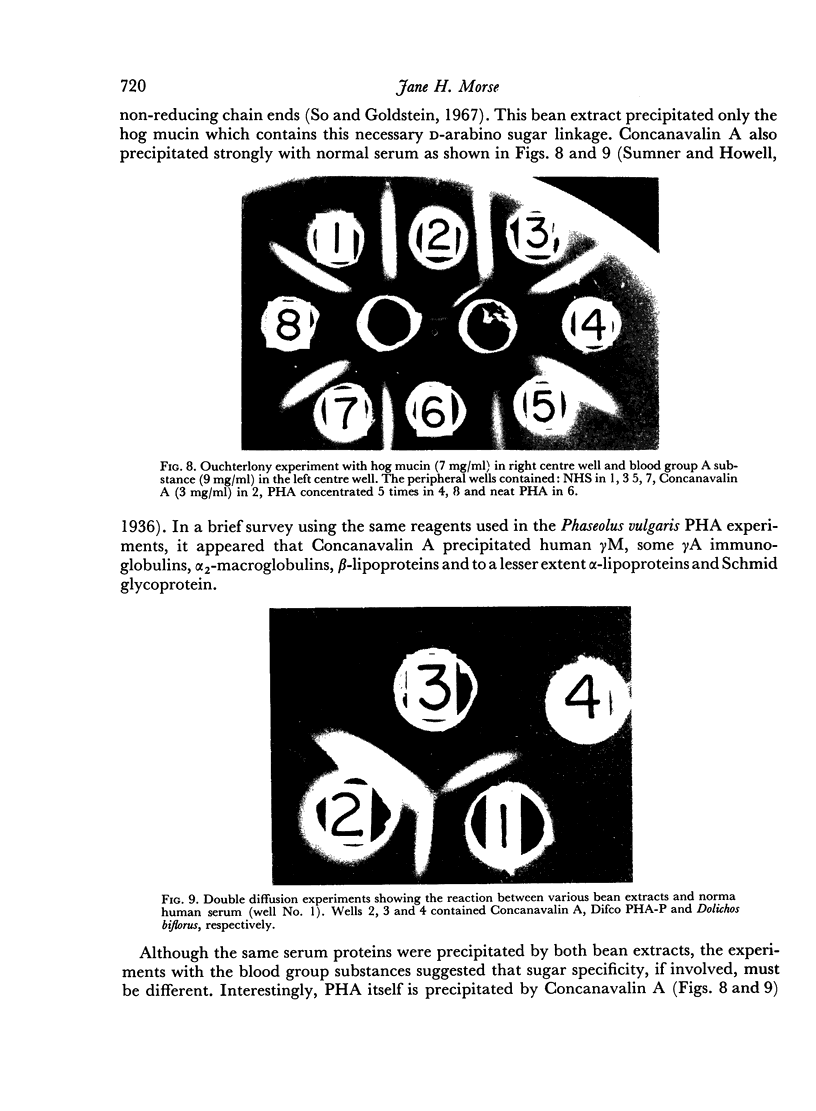
Concanavalin A, a jack bean agglutinin, precipitated the same proteins which were precipitated by Phaseolus vulgaris PHA but a Dolichos biflorus extract did not react with human serum. Incomplete chemical studies of this interaction suggested that even though the same serum proteins were precipitated by Concanavalin A and PHA, the sugar specificity, if involved, is different for both lectins.
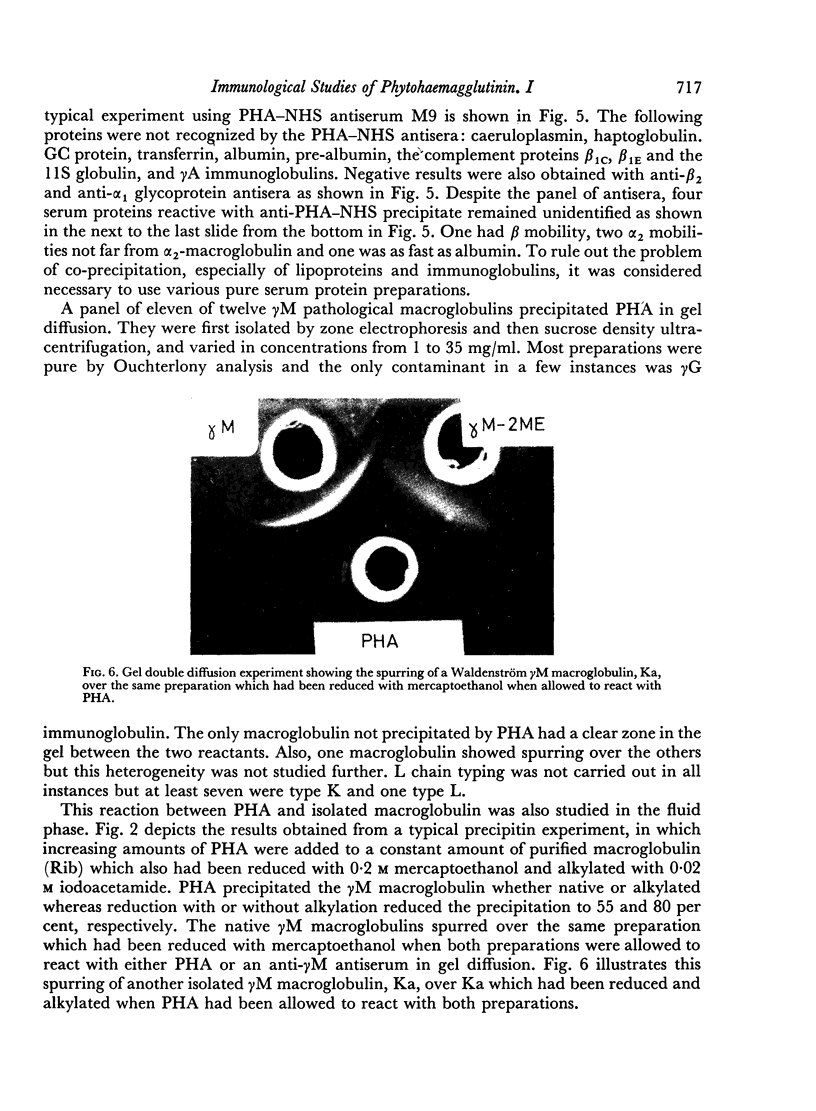
Incomplete studies of the component or components of PHA involved in the PHA—NHS interaction suggested that three cathodally migrating proteins were recognized by both the antisera to PHA and to the precipitate formed by PHA and normal human serum.
Full text
PDF


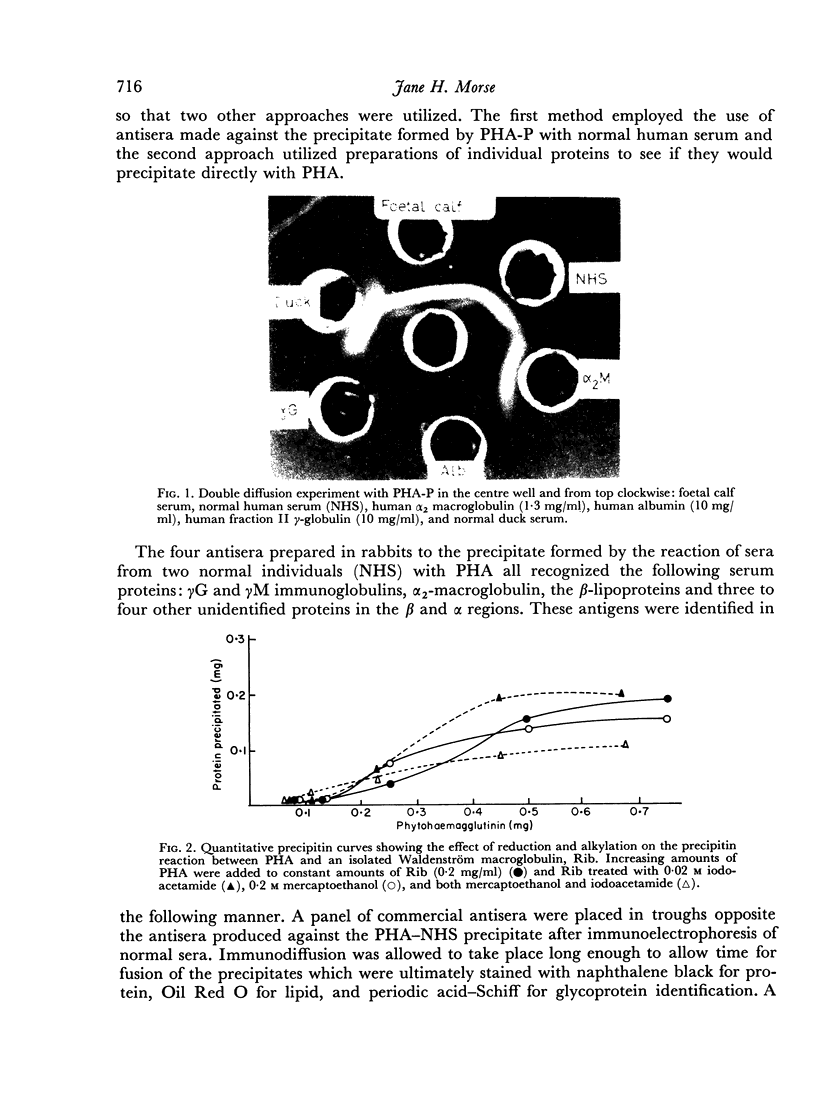
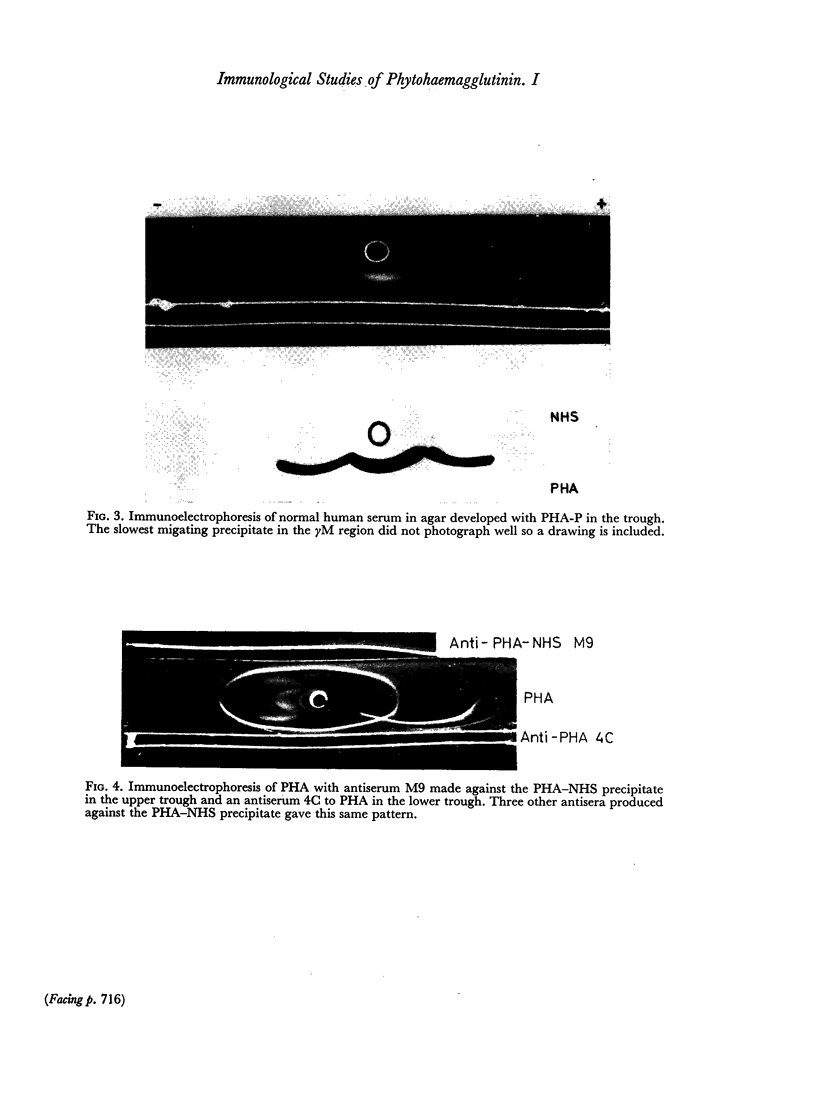





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKMAN L. Effect of phytohaemagglutinin on human serum and cell proteins. Nature. 1962 Aug 11;195:582–583. doi: 10.1038/195582a0. [DOI] [PubMed] [Google Scholar]
- BERMAN L., STULBERG C. S. Primary cultures of macrophages from normal human peripheral blood. Lab Invest. 1962 Dec;11:1322–1331. [PubMed] [Google Scholar]
- Berberg H., Woodruff J., Hirschhorn R., Gesner B., Miescher P., Silber R. Phytohemagglutinin: inhibition of the agglutinating activity by N-acetyl-D-galactosamine. Science. 1966 Nov 25;154(3752):1019–1020. doi: 10.1126/science.154.3752.1019. [DOI] [PubMed] [Google Scholar]
- Börjeson J., Chessin L. N., Landy M. Dissociation of leukagglutinating and transforming properties of phytohemagglutinin by the coating of lymphocytes with Vi polysaccharide. Int Arch Allergy Appl Immunol. 1967;31(2):184–194. doi: 10.1159/000229866. [DOI] [PubMed] [Google Scholar]
- Cooperband S. R., Green J. A., Kennedy M. A., Grant M. M. Dissociation and inhibition of the stimulatory effect of phytohaemagglutinin on protein and DNA synthesis in human lymphocyte cultures. Nature. 1967 Jun 17;214(5094):1240–1241. doi: 10.1038/2141240a0. [DOI] [PubMed] [Google Scholar]
- EDELMAN G. M., KUNKEL H. G., FRANKLIN E. C. Interaction of the rheumatoid factor with antigen-antibody complexes and aggregated gamma globulin. J Exp Med. 1958 Jul 1;108(1):105–120. doi: 10.1084/jem.108.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland N. H., Holland P. Haemagglutinating, precipitating and lymphocyte-stimulating factors of phytohaemagglutinin. Nature. 1965 Sep 18;207(5003):1307–1308. doi: 10.1038/2071307a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYCETTE R. R., PEARMAIN G. E. FURTHER OBSERVATIONS ON ANTIGEN-INDUCED MITOSIS. Lancet. 1963 Aug 24;2(7304):386–386. doi: 10.1016/s0140-6736(63)93063-0. [DOI] [PubMed] [Google Scholar]
- MARSHALL W. H., NORINS L. C. ANTIGENIC PROPERTIES OF THE EXTRACT OF PHASEOLUS VULGARIS SEEDS (PHYTOHAEMAGGLUTININ) ROUTINELY USED IN LEUCOCYTE CULTURES. Aust J Exp Biol Med Sci. 1965 Jun;43:213–228. doi: 10.1038/icb.1965.20. [DOI] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J. A new supporting medium for preparative electrophoresis. Scand J Clin Lab Invest. 1960;12:33–37. [PubMed] [Google Scholar]
- NAKAMURA S., TANAKA K., MURAKAWA S. Specific protein of legumes which reacts with animal proteins. Nature. 1960 Oct 8;188:144–145. doi: 10.1038/188144b0. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- RIGAS D. A., OSGOOD E. E. Purification and properties of the phytohemagglutinin of Phaseolus vulgaris. J Biol Chem. 1955 Feb;212(2):607–615. [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IV. Application of the quantitative precipitin method to polysaccharide-concanavalin A interaction. J Biol Chem. 1967 Apr 10;242(7):1617–1622. [PubMed] [Google Scholar]
- Sumner J. B., Howell S. F. Identification of Hemagglutinin of Jack Bean with Concanavalin A. J Bacteriol. 1936 Aug;32(2):227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Nordman C. T., Gräsbeck R. Separation of lymphocyte-stimulating and agglutinating activities in phytohaemagglutinin (PHA) from Phaseolus vulgaris. Scand J Haematol. 1967;4(1):77–80. doi: 10.1111/j.1600-0609.1967.tb01601.x. [DOI] [PubMed] [Google Scholar]