Abstract
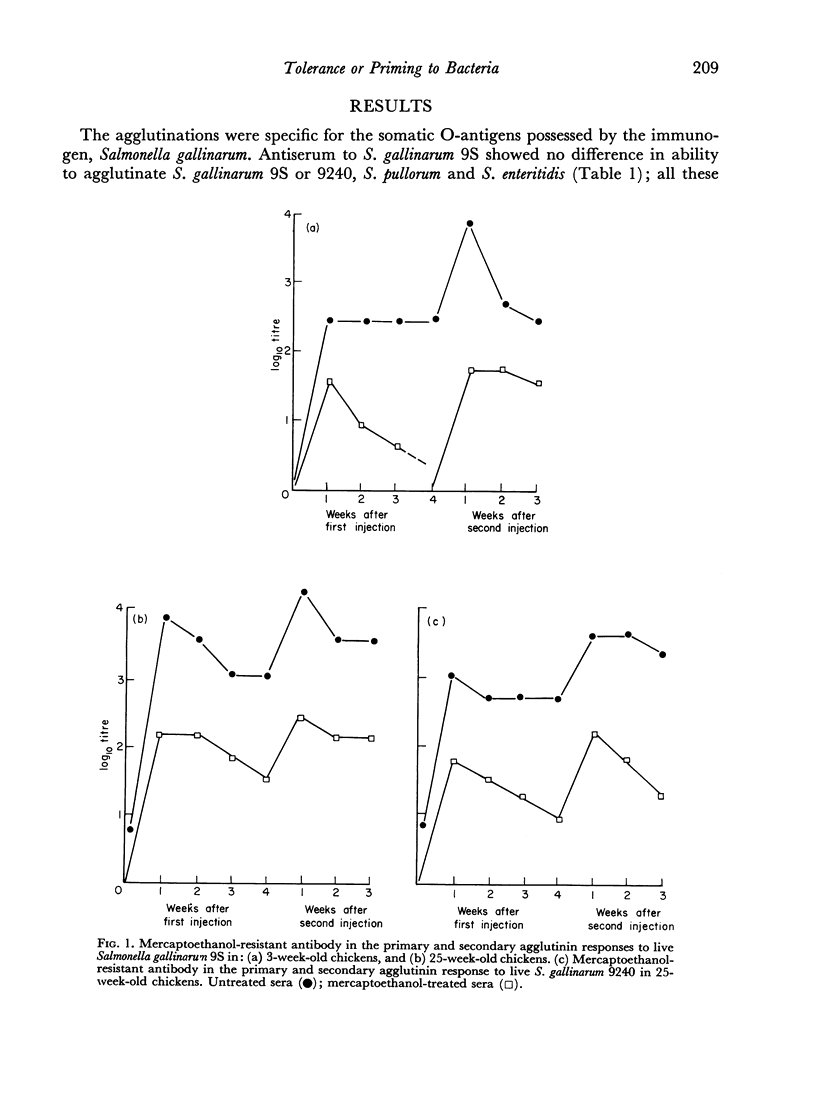
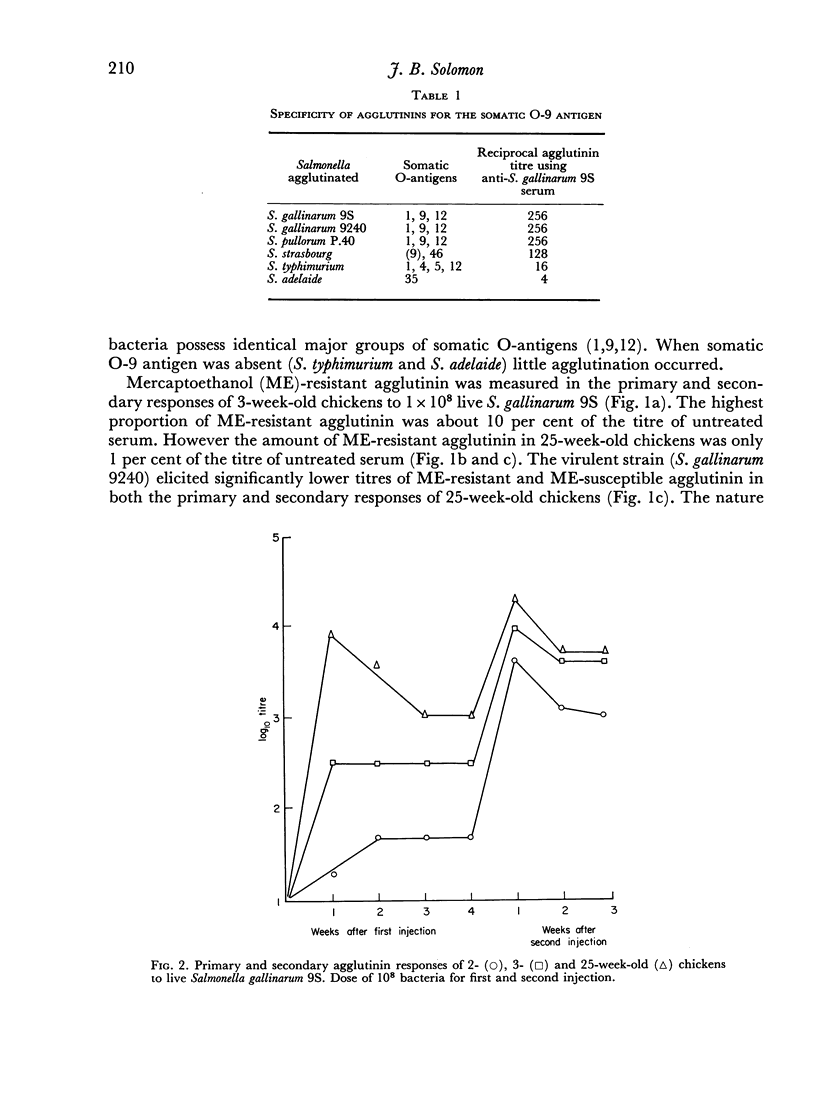
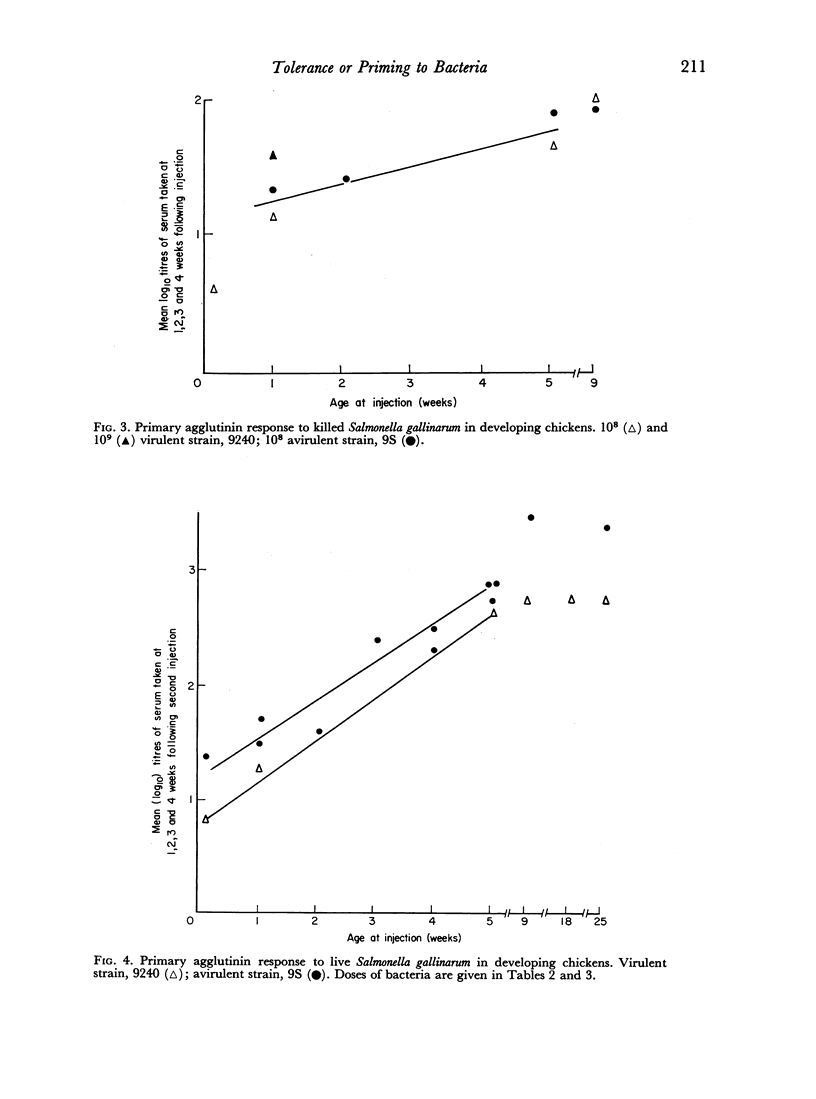
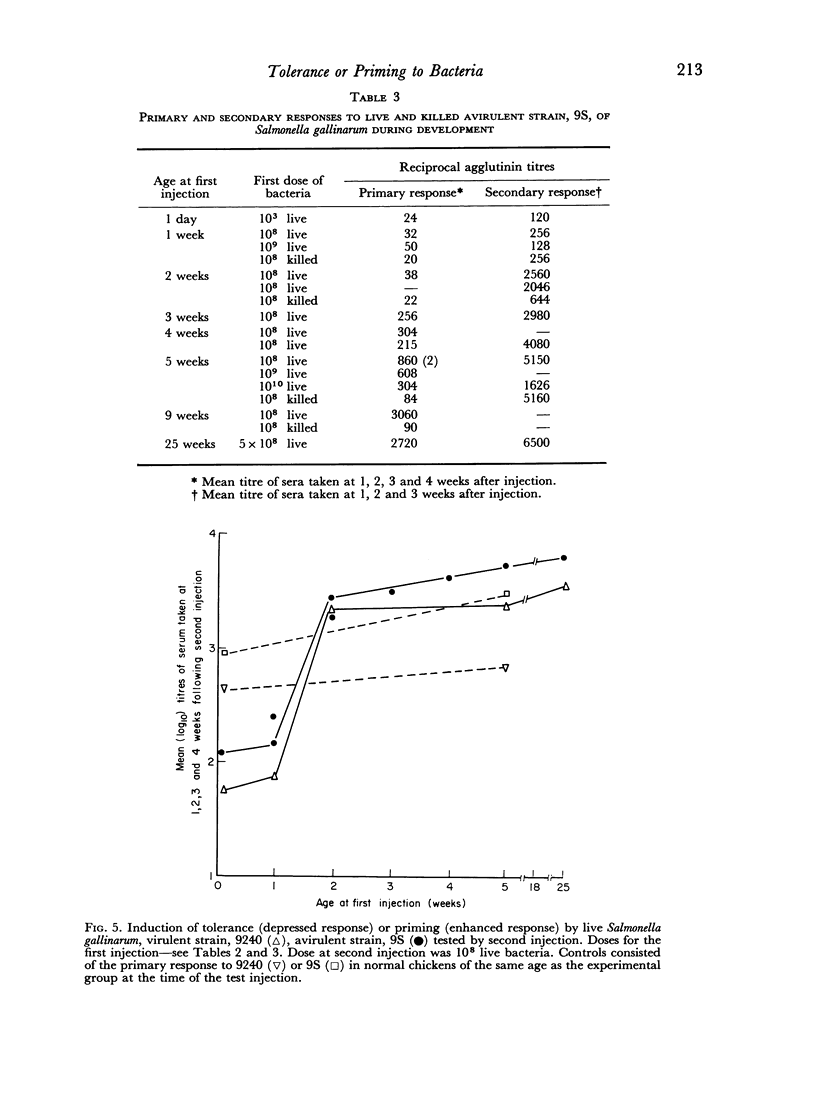
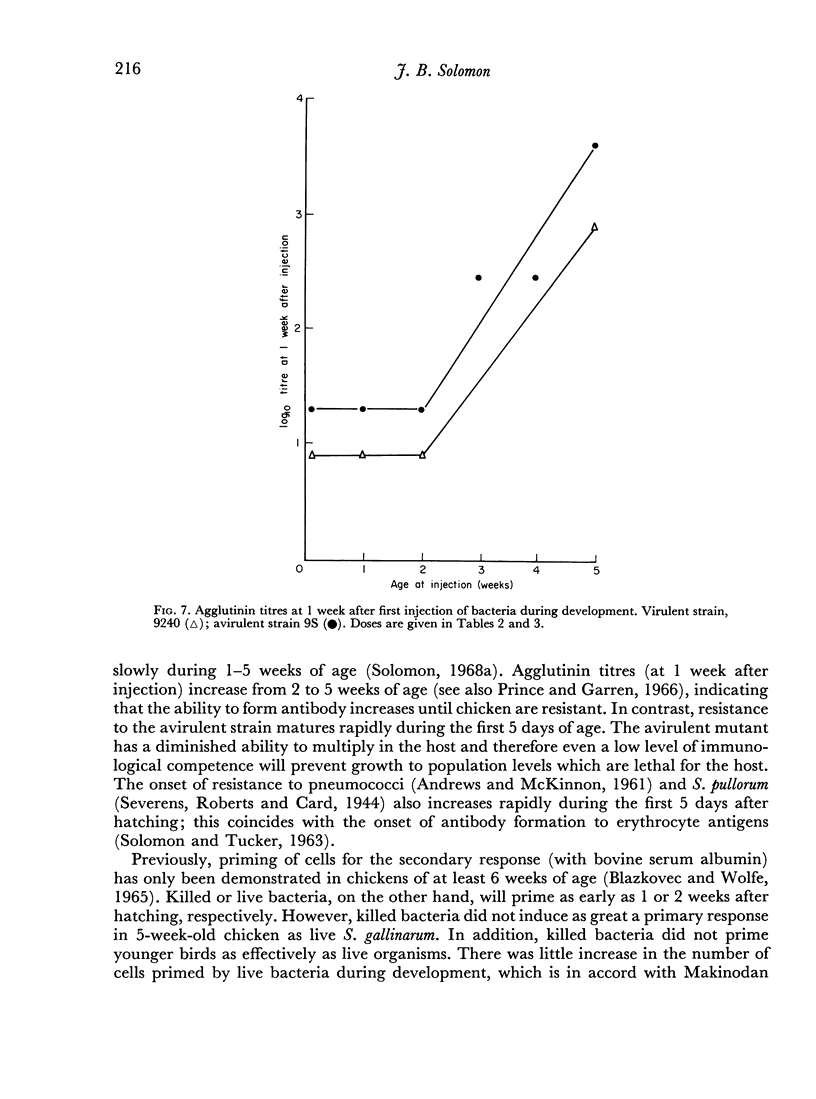
Partial tolerance was induced in cells forming agglutinins by live or killed, virulent or avirulent strains of Salmonella gallinarum in chick embryos, 1-day and 1-week-old chicks. Tolerance was induced by a single injection of only 100–200 live virulent organisms in 1-day-old and 1-week-old chicks. However, 109 avirulent organisms were required to induce a similar degree of tolerance in 1-week-old chicks. The primary agglutinin response paralleled the relative increase in spleen weight during early development and increased only slightly after 5 weeks of age. Priming to live bacteria occurred at 2 weeks of age; the magnitude of the secondary response showed little further increase with age. The induction of partial tolerance in cells producing agglutinins did not increase susceptibility to infection and further emphasizes that agglutinin production is not a protective immune mechanism for S. gallinarum infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREWS E. C., Jr, McKINNON G. E. Studies on the resistance of fowl to pneumococcal infection. Am J Pathol. 1961 Nov;39:579–588. [PMC free article] [PubMed] [Google Scholar]
- BLAZKOVEC A. A., WOLFE H. R. FACTORS AFFECTING THE PRIMARY AND SECONDARY RESPONSES TO BOVINE SERUM ALBUMIN IN CHICKENS. Int Arch Allergy Appl Immunol. 1965;26:80–95. doi: 10.1159/000229556. [DOI] [PubMed] [Google Scholar]
- BUXTON A. Antibody production in avian embryos and young chicks. J Gen Microbiol. 1954 Jun;10(3):398–410. doi: 10.1099/00221287-10-3-398. [DOI] [PubMed] [Google Scholar]
- Delhanty J. J., Solomon J. B. The nature of antibodies to goat erythrocytes in the developing chicken. Immunology. 1966 Aug;11(2):103–113. [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN H., GABY W. L. Immunologic unresponsiveness to Shigella antigens in chickens. J Immunol. 1960 May;84:441–448. [PubMed] [Google Scholar]
- GOWLAND G., OAKLEY C. L. Acquired immunological tolerance of diphtheria alum-precipitated toxoid in the domestic fowl. J Pathol Bacteriol. 1960 Oct;80:373–378. doi: 10.1002/path.1700800221. [DOI] [PubMed] [Google Scholar]
- Gowland G., Hobbs G., Byers H. Attempts to suppress agglutinin production to a bacterial antigen (Pseudomonas sp.) in the neonatal rabbit. J Pathol Bacteriol. 1965 Oct;90(2):443–457. [PubMed] [Google Scholar]
- HIGNETT P. G., NAGY L. K. EFFECT OF EXPOSURE OF VERY YOUNG CALVES TO VIRULENT BRUCELLA ABORTAS ON THEIR SEROLOGICAL RESPONSE TO RE-INFECTION BY THE SAME ORGANISM AT 6 MONTHS OF AGE. Nature. 1964 Jan 11;201:204–204. doi: 10.1038/201204a0. [DOI] [PubMed] [Google Scholar]
- MITCHISON N. A. INDUCTION OF IMMUNOLOGICAL PARALYSIS IN TWO ZONES OF DOSAGE. Proc R Soc Lond B Biol Sci. 1964 Dec 15;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- Makinodan T., Albright J. F. Proliferative and differentiative manifestations of cellular immune potential. Prog Allergy. 1967;10:1–36. [PubMed] [Google Scholar]
- NAGY L. K. The effect of Brucella infection of young lambs on their serological responsiveness to the same antigens later in life. Immunology. 1963 Jan;6:48–63. [PMC free article] [PubMed] [Google Scholar]
- Prince W. R., Garren H. W. An investigation of the resistance of white leghorn chicks to Salmonella gallinarum. Poult Sci. 1966 Nov;45(6):1149–1153. doi: 10.3382/ps.0451149. [DOI] [PubMed] [Google Scholar]
- SMITH H. W. The use of live vaccines in experimental Salmonella gallinarum infection in chickens with observations on their interference effect. J Hyg (Lond) 1956 Sep;54(3):419–432. doi: 10.1017/s0022172400044685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLOMON J. B. Relationship between splenomegaly, runting and tolerance to skin homografts in chicken. Nature. 1962 Nov 10;196:558–560. doi: 10.1038/196558a0. [DOI] [PubMed] [Google Scholar]
- SOLOMON J. B., TUCKER D. F. ONTOGENESIS OF IMMUNITY TO ERYTHROCYTE ANTIGENS IN THE CHICK. Immunology. 1963 Nov;6:592–601. [PMC free article] [PubMed] [Google Scholar]
- STERZL J., TRNKA Z. Effect of very large doses of bacterial antigen on antibody production in newborn rabbits. Nature. 1957 May 4;179(4566):918–919. doi: 10.1038/179918a0. [DOI] [PubMed] [Google Scholar]
- Solomon J. B. Immunity to Salmonella gallinarum during ontogeny of the chicken. 3. Bactericidal antibody. Immunology. 1968 Aug;15(2):219–226. [PMC free article] [PubMed] [Google Scholar]
- Solomon J. B. Immunity to Salmonella gallinarum during ontogeny of the chicken. I. Onset of resistance to infection; the minor role of opsonins. Immunology. 1968 Aug;15(2):197–206. [PMC free article] [PubMed] [Google Scholar]
- Solomon J. B. Immunity to salmonella gallinarum during ontogeny of the chicken. IV. Immunocyto-adherence and a cytophilic antibody. Immunology. 1968 Aug;15(2):227–236. [PMC free article] [PubMed] [Google Scholar]


