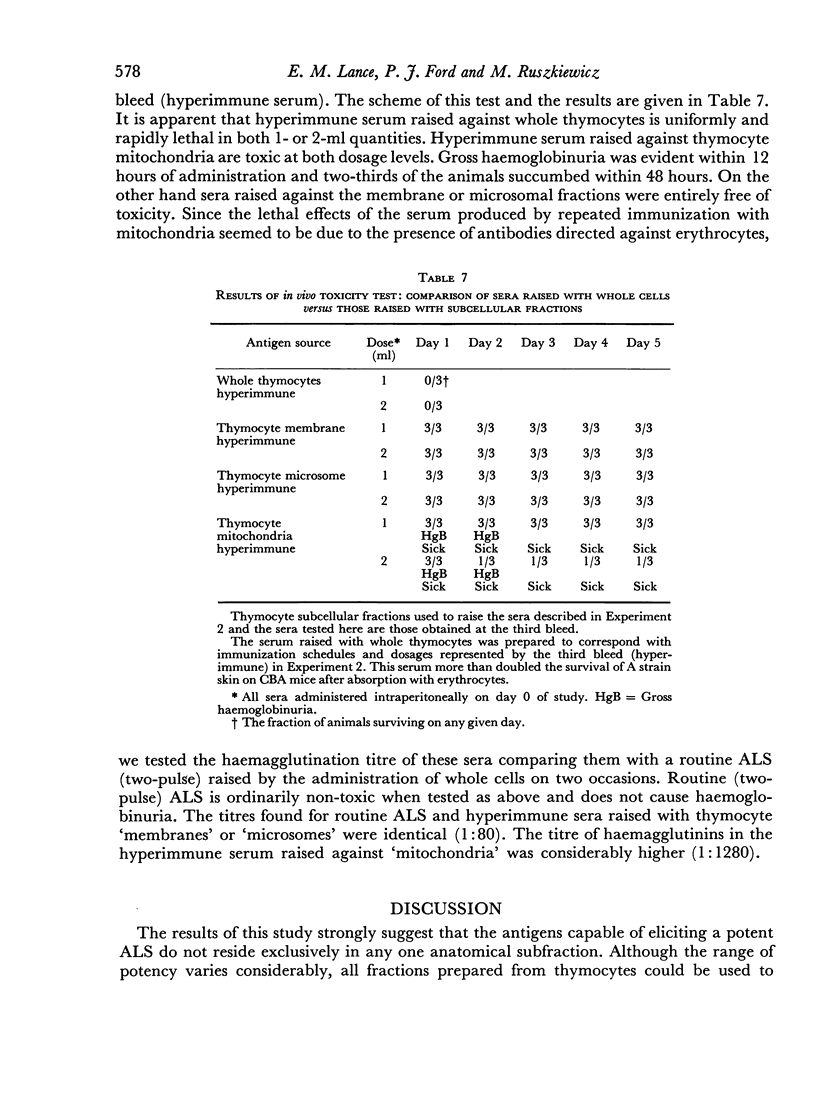
Abstract
Subcellular preparations of mouse thymocytes were compared with respect to their power to raise in rabbits antisera which prolonged the survival of skin homografts. Of the fractions studied thymocyte `membrane' gave the most consistently good results, whereas thymocyte `mitochondria' gave rise to sera which were toxic in high dose. Whole mouse liver homogenate or liver subcellular fractions did not elicit potent antisera. It is concluded that thymocyte `membrane' best fulfils the requirements for an ideal material for immunization thus far studied and provides a convenient starting point for further purification.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EARL D. C., KORNER A. THE ISOLATION AND PROPERTIES OF CARDIAC RIBOSOMES AND POLYSOMES. Biochem J. 1965 Mar;94:721–734. doi: 10.1042/bj0940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Levey R. H., Medawar P. B. Nature and mode of action of antilymphocytic antiserum. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1130–1137. doi: 10.1073/pnas.56.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., SELIGMAN A. M. Evidence for the specificity of esterase and lipase by the use of three chromogenic substrates. J Biol Chem. 1949 Nov;181(1):343–355. [PubMed] [Google Scholar]
- NEVILLE D. M., Jr The isolation of a cell membrane fraction from rat liver. J Biophys Biochem Cytol. 1960 Oct;8:413–422. doi: 10.1083/jcb.8.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. The formation, distribution and function of ribosomes and microsomal membranes during induced amphibian metamorphosis. Biochem J. 1967 Nov;105(2):783–801. doi: 10.1042/bj1050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIGZELL H. QUANTITATIVE TITRATIONS OF MOUSE H-2 ANTIBODIES USING CR-51-LABELLED TARGET CELLS. Transplantation. 1965 May;3:423–431. doi: 10.1097/00007890-196505000-00011. [DOI] [PubMed] [Google Scholar]



