Abstract
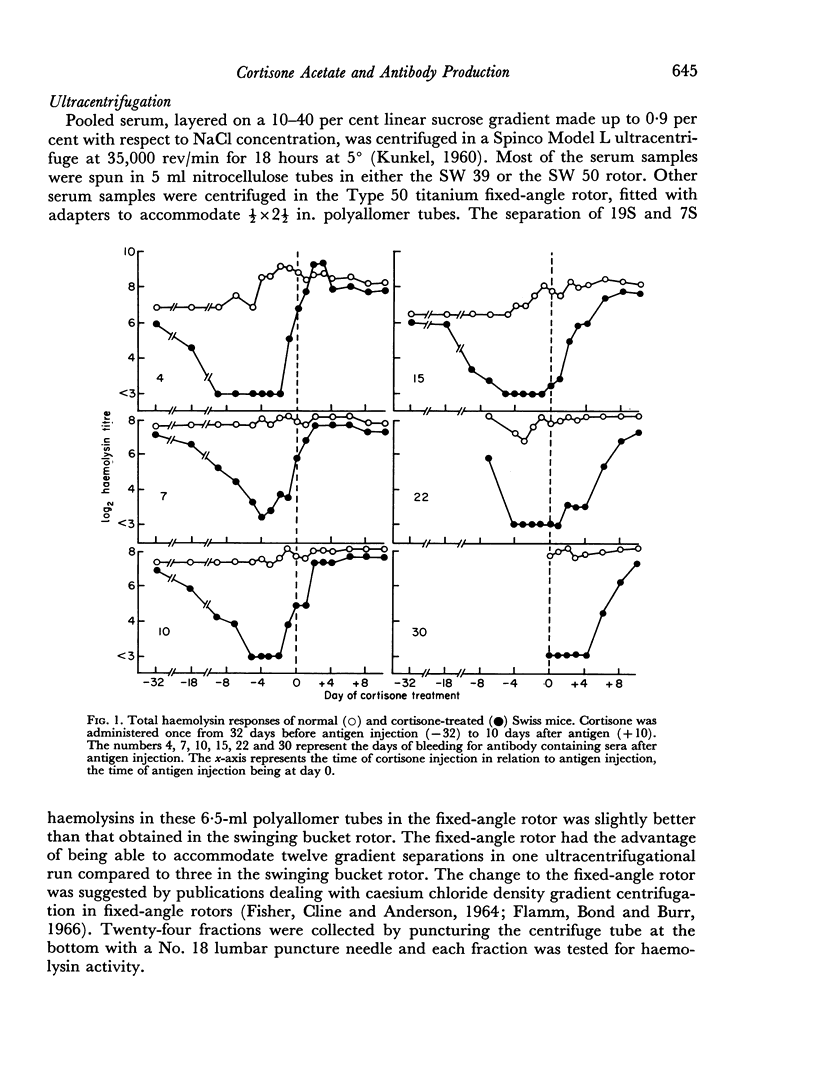
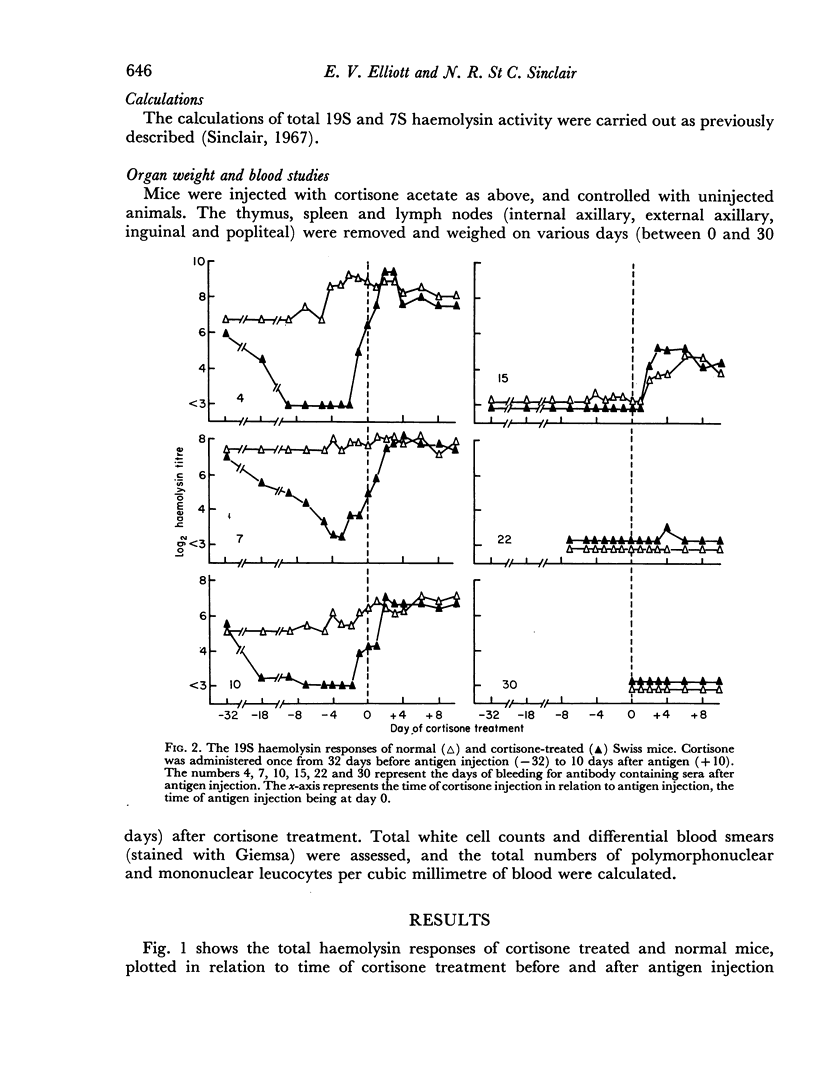
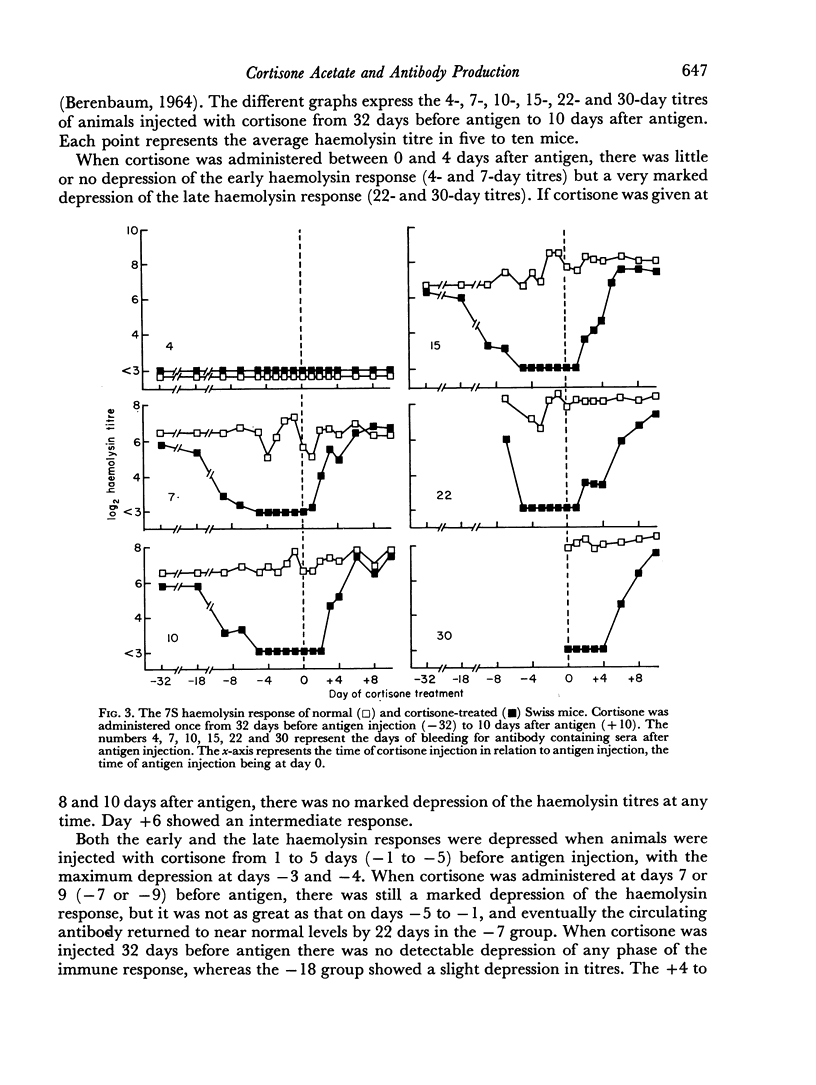
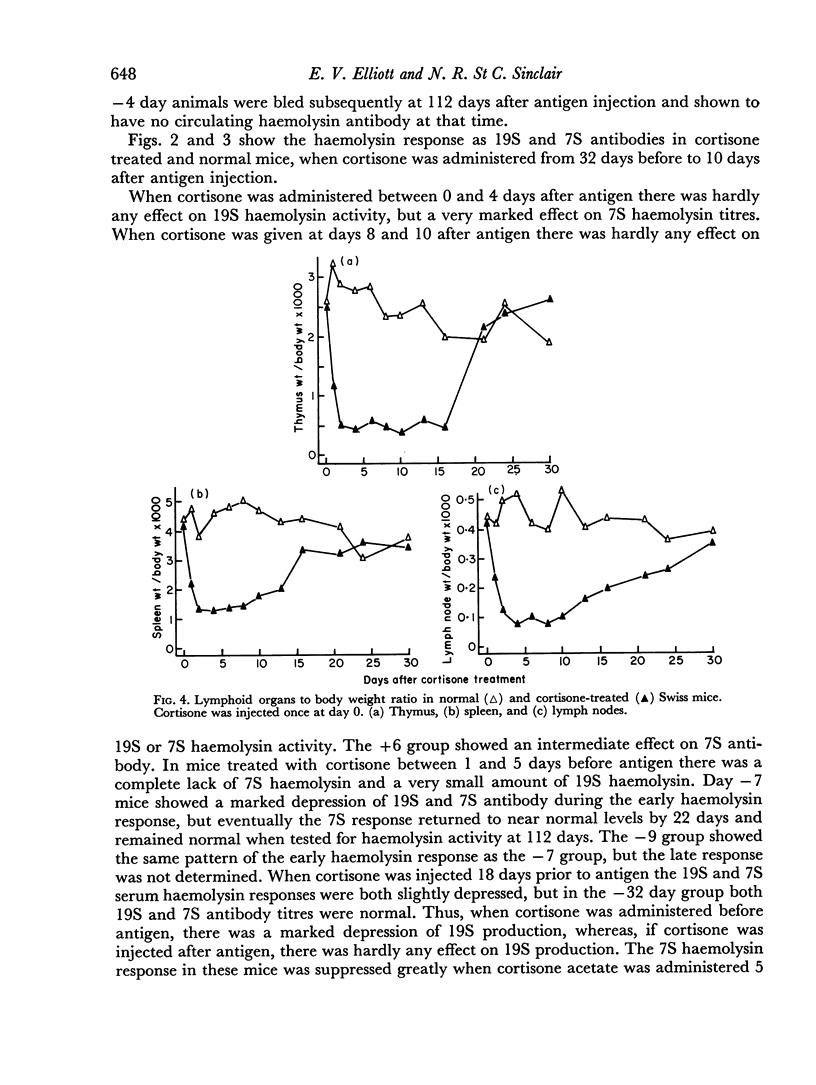
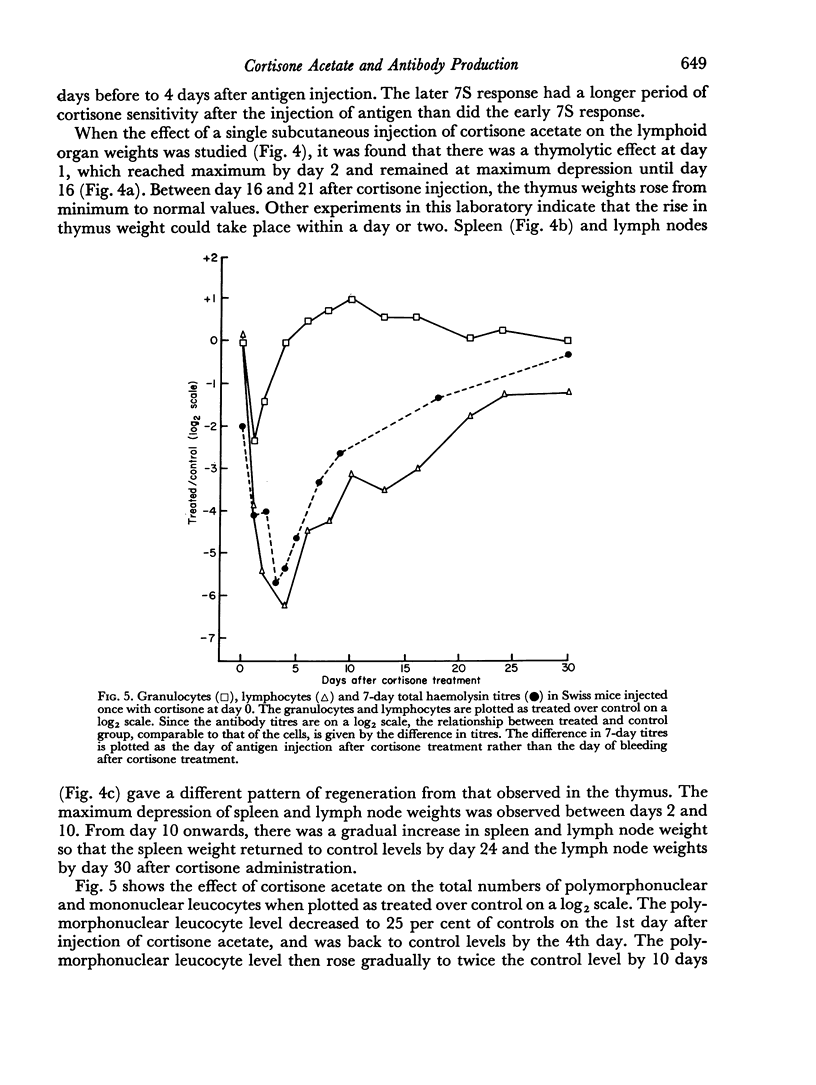
A single subcutaneous injectio of cortisone acetate (400–500 mg/kg body weight) depressed the serum haemolysin response of adult Swiss mice to sheep erythrocytes when administered near the time of antigen injection. The greatest suppression of the early haemolysin response occurred when cortisone was injected 3–4 days prior to antigen, while the late antibody response (20–30 days after antigen injection) was markedly decreased when cortisone was given up to 4 days after antigen. Cortisone depressed both 19S and 7S haemolysin when given prior to antigen, but depressed only the 7S antibody when administered after antigen. An attempt was made to correlate the depression of the immune response with the decrease in lymphoid tissue following cortisone treatment and a striking correlation was observed between the number of circulating lymphocytes at the time of antigen injection and the 7 day titres of total and 19S antibody.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER F. L. STUDIES ON MOUSE ANTIBODIES. I. THE RESPONSE TO SHEEP RED CELLS. J Immunol. 1965 Jul;95:26–38. [PubMed] [Google Scholar]
- BERENBAUM M. C. EFFECTS OF CARCINOGENS ON IMMUNE PROCESSES. Br Med Bull. 1964 May;20:159–164. doi: 10.1093/oxfordjournals.bmb.a070311. [DOI] [PubMed] [Google Scholar]
- BERGLUND K., FAGRAEUS A. A biological factor inhibiting the effect of cortisone on antibody formation. Nature. 1956 Feb 4;177(4501):233–234. doi: 10.1038/177233a0. [DOI] [PubMed] [Google Scholar]
- BERGLUND K., FAGRAEUS A. Studies on the effect of homologous spleen cells on antibody formation in cortisone treated rats. Acta Pathol Microbiol Scand. 1961;52:321–329. doi: 10.1111/j.1699-0463.1961.tb03200.x. [DOI] [PubMed] [Google Scholar]
- BERGLUND K. Studies on factors which condition the effect of cortisone on antibody production. 3. The significance of time of hormone administration in primary agglutinin response to S. typhi H. Acta Pathol Microbiol Scand. 1956;38(5):403–415. doi: 10.1111/j.1699-0463.1956.tb04660.x. [DOI] [PubMed] [Google Scholar]
- BERGLUND K. Studies on factors which condition the effect of cortisone on antibody production. I. The significance of time of hormone administration in primary hemolysin response. Acta Pathol Microbiol Scand. 1956;38(4):311–328. doi: 10.1111/j.1699-0463.1956.tb01707.x. [DOI] [PubMed] [Google Scholar]
- BERGLUND K. Studies on factors which condition the effect of cortisone on antibody production. II. The significance of the dose of antigen in primary hemolysin response. Acta Pathol Microbiol Scand. 1956;38(4):329–338. doi: 10.1111/j.1699-0463.1956.tb01708.x. [DOI] [PubMed] [Google Scholar]
- Dietrich F. M. The immune response to heterologous red cells in mice. Immunology. 1966 Apr;10(4):365–376. [PMC free article] [PubMed] [Google Scholar]
- Dukor P., Dietrich F. M. Chemical suppression of immune responses in thymectomized mice. Int Arch Allergy Appl Immunol. 1967;32(2):131–148. doi: 10.1159/000229923. [DOI] [PubMed] [Google Scholar]
- FAGRAEUS A., BERGLUND K. Inhibition of the effect of cortisone on antibody formation by heterologous spleen cells. J Immunol. 1961 Jul;87:49–55. [PubMed] [Google Scholar]
- FISHER W. D., CLINE G. B., ANDERSON N. G. DENSITY GRADIENT CENTRIFUGATION IN ANGLE-HEAD ROTORS. Anal Biochem. 1964 Dec;9:477–482. doi: 10.1016/0003-2697(64)90209-x. [DOI] [PubMed] [Google Scholar]
- ISHIDATE M., METCALF D. THE PATTERN OF LYMPHOPOIESIS IN THE MOUSE THYMUS AFTER CORTISONE ADMINISTRATION OR ADRENALECTOMY. Aust J Exp Biol Med Sci. 1963 Dec;41:637–649. doi: 10.1038/icb.1963.53. [DOI] [PubMed] [Google Scholar]
- Jaroslow B. N., Nossal G. J. Effect of x-irradiation on antigen localization in lymphoid follicles. Aust J Exp Biol Med Sci. 1966 Dec;44(6):609–627. doi: 10.1038/icb.1966.60. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Sahiar K., Schwartz R. S. The immunoglobulin sequence. I. Arrest by 6-mercaptopurine and restitution by antibody, antigen or splenectomy. J Immunol. 1965 Aug;95(2):345–354. [PubMed] [Google Scholar]
- Sinclair N. R. A comparison of primary and secondary haemolysin responses to sheep erythrocytes in neonatally thymectomized, sham-thymectomized and normal Swiss mice. A time-course study of total, 19S and 7S antibody. Immunology. 1967 May;12(5):559–564. [PMC free article] [PubMed] [Google Scholar]
- Sinclair N. R. Effects of neonatal thymectomy on the haemolysin response to sheep erythrocytes in Swiss albino mice. A time-course study of total, 19S and 7S antibody. Immunology. 1967 May;12(5):549–557. [PMC free article] [PubMed] [Google Scholar]
- Williams G. M. Antigen localization in lymphopenic states. I. Localization pattern following chronic thoracic duct drainage. Immunology. 1966 Nov;11(5):467–474. [PMC free article] [PubMed] [Google Scholar]
- Williams G. M. Antigen localization in lymphopenic states. II. Further studies on whole body x-irradiation. Immunology. 1966 Nov;11(5):475–488. [PMC free article] [PubMed] [Google Scholar]


