Abstract
A subset of patients suffering from familial amyotrophic lateral sclerosis (FALS) exhibit point mutations in the gene encoding Cu-Zn superoxide dismutase [superoxide:superoxide oxidoreductase, EC 1.15.1.1 (SOD)]. The human wild-type and five FALS Sod mutant transgenes were introduced into the fruit fly, Drosophila melanogaster, in a Cu-Zn Sod null background. Sod null flies had dramatically decreased life span, glutathione and methionine content, fertility, locomotor activity, and resistance to hyperoxic stress, compared with wild-type controls. All of these phenotypic manifestations were rescued fully by a single human wild-type allele, expressing 5–10% of wild-type SOD activity. Full recovery of wild-type life span was also observed when human mutant and wild-type alleles were placed together in the fly Sod null background. The FALS Sod mutations alone caused a recessive phenotype, usually involving low or undetectable levels of SOD activity, in which: (i) full restoration of the wild-type phenotype was observed among young adults, and (ii) older adults exhibited a sudden increase in oxidative stress, accompanied by physiological impairment of abrupt onset, and followed by premature death. Thus, the minimal SOD activity associated with the FALS Sod mutations appears to determine longevity, not by chronically increasing oxidative stress, but by limiting the time in which a viable redox environment can be maintained. However, the dominant gain of function by mutant SOD, which occurs in human patients and in the transgenic mouse model of FALS, is not observed in Drosophila.
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, is a rapidly progressive human neurological disorder, in which loss of motor neurons leads to paralysis and death (1). Approximately 10% of ALS cases are familial (FALS), and 10–15% of FALS patients have point mutations in the gene encoding Cu-Zn superoxide dismutase [superoxide:superoxide oxidoreductase, EC 1.15.1.1 (SOD)] (2, 3). Most FALS Sod mutations lead to attenuation of enzymatic activity, but a varying amount of activity is retained (4). The mutant SOD enzymes exhibit conformational changes that decrease their zinc-binding affinity and increase free radical production (5, 6).
Transgenic mice expressing human FALS-associated Sod alleles exhibit phenotypes resembling human ALS, involving the formation of SOD-containing inclusions in neurons and astrocytes, spinal motor neuron loss leading to the rapid onset of paralysis, and death in early to mid-adult life (7, 8). The timing of disease onset and progression is independent of levels of human or mouse wild-type SOD activity, showing that FALS Sod mutations induce a dominant “gain-of-function,” rather than a simple loss of enzymatic activity. However, the role of oxidative stress in this gain of function remains unclear. Administration of SOD/catalase mimetic compounds prevented the accumulation of some oxidative damage byproducts in FALS SOD transgenic mice but had very limited effects on disease onset and survival times (9).
The relationships among SOD activity, oxidative stress, and longevity have been studied extensively in the fruit fly, Drosophila melanogaster (10–12), but only one study examined the effects of a human FALS Sod mutant allele in Drosophila (13). Expression of this allele led to extension of life span, which paralleled the results of human wild-type SOD expression in Drosophila motor neurons, but did not suggest a plausible model for the shortening of life span that occurs in ALS patients. However, expression of the allele was restricted to motor neurons, a cell type that normally has very low SOD activity in Drosophila (14), and similar neuron-specific expression also failed to produce an ALS-like phenotype in transgenic mice (15). In both human FALS and the mouse model of FALS, the mutant enzyme is expressed ubiquitously, even though pathology is restricted to motor neurons. Thus, notwithstanding the utility of Drosophila as a model for other human neurological disorders (16), the possibility of a Drosophila model of FALS remains underexplored.
In the present study, the human wild-type and five mutant FALS Sod alleles, governed by the Drosophila native Sod cis regulatory sequences, were placed in a Drosophila Cu-Zn Sod null background. Subsequently, the wild-type and mutant alleles were placed together in the fly Sod null background. The hypotheses tested and confirmed in this study were that: (i) only minimal levels of SOD activity are required to sustain a normal life span in Drosophila, (ii) expression of FALS Sod shortens the life span, in comparison with human wild-type Sod, and (iii) the decrease in life span is associated with increased oxidative stress and decreased motor performance of abrupt onset. However, the decrease in life span was recessive, demonstrating that the effects of FALS Sod expression in Drosophila differ from those in humans.
Materials and Methods
FALS Sod Gene Construction.
The human wild-type and G37R, G41D, G93C, and I113T mutant Sod cDNAs were amplified by PCR by using the primers 5′-AATTCATTCGAAATGGCGACGAAGGCCGTGTG-3′ (forward) and 5′-TTGACTTGCTCAGCTTATTGGGCGATCCCAATTAC-3′ (reverse). The A4V mutation was introduced as a C→T transition at position +14 in the forward primer. The underlined sequences correspond to BstB I and Blp I sites, respectively, and are positioned immediately flanking the start and stop codons (boldface) of the human Sod coding sequence. The PCR product was subcloned into the Drosophila genomic Sod fragment described in ref. 11, after digestion with BstB I and Blp I. The resulting constructs contained an exact fusion of the Drosophila 5′ untranslated sequence with the human coding sequence. The 3′ fusion lay 68 base pairs upstream from the Drosophila Sod stop codon. The presence of the mutations was confirmed by sequencing, and the constructs were then cloned into the EcoR I site of the pCaSpeR vector (17). The unmodified Drosophila genomic Sod fragment in the EcoR I site of pCaSpeR was used as a positive control.
Transgenic Flies.
Microinjection of Drosophila embryos was performed as described (11, 18) by using either y w or y w; ry506 Sb P[ry+ Δ2–3](99B)/TM6, Ubx recipient strains. Southern analysis and chromosome mapping confirmed the presence of a single transgene on chromosome 2, after microinjection or transient remobilization in crosses to the strain CyO/Sp; Sb Δ2–3/TM6, Ubx. Transgenes on chromosome 2 were then crossed into a Drosophila Sod null background, by using dominantly marked balancer chromosomes and the Sodx16 and Sodx39 deletion alleles (10) to generate stocks with the genotype y w; P[FALS Sod, w+]/CyO; Sodx16/TM3, Sb Ser. Stocks in which the P element failed to outcompete the CyO balancer were excluded from subsequent experiments to decrease the likelihood of insertional position effects involving neighboring loci. Male flies from stocks with homozygous P elements were outcrossed to Sodx39/TM3, Sb Ser virgin females, yielding male P[FALS Sod, w+]/+; Sodx16/x39 progeny for use in experiments. These flies were designated SodA4V; Sodx16/x39, etc., or Sodx39/x16 if the Sodx16 and Sodx39 alleles were switched in the parental stocks. For life spans involving both wild-type and mutant alleles, the male flies were crossed with y w; P[Sod+, w+]; Sodx39/TM3, Sb Ser virgin females, yielding male y w; P[FALS Sod, w+]/P[Sod+, w+]; Sodx16/x39 progeny.
Near the end of the study, the presence of the mutations was reconfirmed by PCR amplification and sequencing of genomic DNA from each transgenic stock.
Drosophila Life Span and Physiology.
Life spans and hyperoxic stress experiments were performed as described (11), except that survivors in life-span studies were pooled when total numbers in multiple vials were five or fewer. Geotactic activity was determined by using flies enclosed individually within narrow plastic cylinders, which prevented flight. Fertility experiments were performed by crossing groups of five virgin females with five wild-type males, 2–3 days after collection. The male flies were progeny from an outbreeding cross of y w males with wild-type females. The parental flies were allowed to lay eggs for 1-day intervals initially, and 2- to 3-day intervals at older ages. Progeny were counted after full eclosion, typically 12–13 days after egg laying.
SOD Assay.
SOD activity was assayed on nitroblue tetrazolium activity gels, essentially as described (19). Activity was quantified by densitometric scanning, with background subtraction, and expressed as a percentage of wild-type activity determined on the same gel, or based on Sodx39/+ activity as a percentage of wild type.
Antioxidant Status.
The quantities of glutathione and methionine were determined by using a Shimadzu Class-VP high-performance liquid chromatography system, largely as described (20), except that the mobile phase contained 25 mM sodium phosphate, pH 2.7, 0.15 mM octanesulfonic acid, and 2% acetonitrile (vol/vol), and separation times were 30–35 min. Electrochemical detection was performed with analytical cell settings of +750 and +875 mV.
Results
Minimal Levels of Wild-Type SOD Activity Sustain Life Span in Drosophila.
To establish controls for comparison with FALS Sod mutants, five Drosophila wild-type Sod (dSod) and seven human wild-type Sod (hSod) transgenic lines were constructed. Negative controls [five (Sod−) lines] contained vector sequences with no Sod insert. The transgenes were then crossed into an endogenous Sod null background (Sodx16/x39). Additional control lines were generated by combining one, both, or neither of the Sodx16 and Sodx39 alleles in untransformed flies.
In most lines expressing a single dSod transgene in a Sod null background (dSod; Sodx16/x39), the level of SOD activity was reduced by ≈50% in comparison with wild-type flies, but all of these lines lived at least as long as the wild-type flies (Table 1, Figs. 1 and 2). The (Sod−); Sodx16/x39 flies had no detectable SOD activity and, in two replicate experiments, their life spans were decreased by 77% and 88% in comparison with the dSod; Sodx16/x39 mean, consistent with the findings of other investigators (23).
Table 1.
Life spans and SOD activities of individual lines expressing FALS Sod cDNAs
| Drosophila line | Life span, days (n): Experiment 1* | Life span, days (n): Experiment 2* | SOD activity (% wild type)† |
|---|---|---|---|
| Sod+/+ | ND | 59.0 (194) | 100 |
| Sodx39/+ | 73.6 (199) | 68.2 (193) | 49 |
| Sodx16/+ | 56.8 (197) | 54.0 (198) | 49 |
| dSod-1; Sodx16/x39 | 76.1 (198) | 73.3 (198) | 54 |
| dSod-2; Sodx39/x16 | 66.8 (200) | 69.2 (197) | 42 |
| dSod-3; Sodx39/x16 | 60.3 (101) | 67.4 (198) | 96 |
| dSod-4; Sodx16/x39 | 65.3 (193) | 64.7 (193) | 79 |
| dSod-5; Sodx16/x39 | 64.7 (197) | 64.7 (193) | 45 |
| dSod mean | 67.3 (889) | 67.9 (979) | |
| hSod-1; Sodx16/x39 | 67.8 (175) | 69.7 (198) | 7 |
| hSod-2; Sodx16/x39 | 69.3 (199) | 69.7 (202) | 10 |
| hSod-3; Sodx16/x39 | 68.7 (197) | 68.4 (197) | 11 |
| hSod-4; Sodx16/x39 | 64.1 (198) | 67.1 (198) | 4 |
| hSod-5; Sodx16/x39 | 62.7 (197) | 63.3 (199) | 25 |
| hSod-6; Sodx16/x39 | 59.8 (198) | 62.9 (201) | 7 |
| hSod-7; Sodx16/x39 | 36.6 (200) | 50.4 (198) | 2 |
| hSOD mean | 61.1 (1364) | 64.5 (1393) | |
| SodG93C-1; Sodx39/x16 | 51.6 (196) | 69.1 (193) | 12 |
| SodG93C-2; Sodx16/x39 | 66.5 (195) | 67.9 (198) | 14 |
| SodG93C-3; Sodx16/x39 | 55.9 (174) | 60.6 (196) | 15 |
| SodG93C-4; Sodx16/x39 | 37.7 (150) | 50.4 (197) | 6 |
| SodG93C-5; Sodx39/x16 | 47.8 (199) | 46.6 (198) | 15 |
| SodG93Cmean | 52.5 (914) | 58.9 (982) | |
| SodG37R-1; Sodx16/x39 | 54.1 (198) | 59.6 (198) | 1 |
| SodG37R-2; Sodx16/x39 | 38.1 (197) | 46.8 (199) | 0 |
| SodG37R-3; Sodx16/x39 | 27.1 (125) | 36.1 (195) | 0 |
| SodG37Rmean | 41.5 (520) | 47.6 (592) | |
| SodA4V-1; Sodx16/x39 | 23.6 (199) | 31.4 (199) | 1 |
| SodA4V-2; Sodx16/x39 | ND | 29.9 (198) | 0 |
| SodA4V-3; Sodx16/x39 | 17.8 (199) | 24.8 (199) | 0 |
| SodA4V-4; Sodx16/x39 | 20.6 (198) | 24.3 (197) | 0 |
| SodA4V-5; Sodx16/x39 | 11.8 (170) | 22.2 (193) | 0 |
| SodA4V-6; Sodx16/x39 | 13.6 (65) | 20.9 (196) | 2 |
| SodA4Vmean | 18.3 (831) | 25.6 (1182) | |
| SodG41D-1; Sodx16/x39 | 23.5 (199) | 29.6 (196) | 9 |
| SodG41D-2; Sodx16/x39 | 9.9 (174) | 18.7 (200) | 0 |
| SodG41Dmean | 17.2 (373) | 24.1 (396) | |
| SodI113T-1; Sodx16/x39 | 8.9 (148) | 16.8 (195) | 1 |
| SodI113T-2; Sodx16/x39 | 3.7 (9) | 12.2 (30) | 2 |
| SodI113Tmean | 8.6 (157) | 16.1 (225) | |
| (Sod-)-1; Sodx16/x39 | 9.8 (199) | 19.3 (196) | 1 |
| (Sod-)-2; Sodx16/x39 | 8.5 (98) | 16.4 (200) | 1 |
| (Sod-)-3; Sodx16/x39 | 7.6 (102) | 16.0 (200) | 1 |
| (Sod-)-4; Sodx16/x39 | 7.4 (100) | 14.6 (200) | 1 |
| (Sod-)-5; Sodx16/x39 | 6.5 (166) | 12.0 (129) | 1 |
| (SOD-) mean | 8.1 (665) | 15.9 (925) | |
| Sodx16/x39 | 7.3 (116) | 15.5 (175) | 1 |
| Sodx39/x16 | 8.4 (24) | 14.1 (190) | ND |
Mean life spans of individual lines in the two experiments were strongly correlated: r2 = 0.97. Flies were collected 1 day post-eclosion, and flies that died within 1 day of collection were excluded from the analysis. n, number of animals; ND, not determined.
SOD activity is expressed as a percentage of wild type and is the mean of three to eight determinations (n = 2 for SODI113T-2). Activity levels were also determined by using a spectrophotometric assay based on nitroblue tetrazolium reduction (21), with 2% SDS pretreatment to remove Mn SOD. This method confirmed the relationship among dSOD, hSOD-5, and SODG93C activities, but the low levels of SOD activity in most other lines could not be quantified due to residual interferences in the assay.
Figure 1.
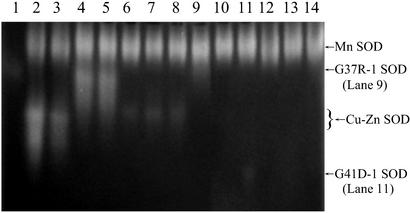
SOD activity in whole-body homogenates of Drosophila. Lanes: 1, bovine erythrocyte SOD (0.06 μg); 2, Sod+/+; 3, Sodx39/+; 4, dSod-1; Sodx16/x39; 5, dSod-5; Sodx16/x39; 6, hSod-1; Sodx16/x39; 7, hSod-3; Sodx16/x39; 8, SodG93C-2; Sodx16/x39; 9, SodG37R-1; Sodx16/x39; 10, SodA4V-1; Sodx16/x39; 11, SodG41D-1; Sodx16/x39; 12, SodI113T-1; Sodx16/x39; 13, (Sod-)-1; Sodx16/x39; 14, Sodx16/x39. The existence of two Drosophila Cu-Zn Sod alleles, resulting in differences in electrophoretic mobility (lanes 2–5), has been discussed previously (22).
Figure 2.
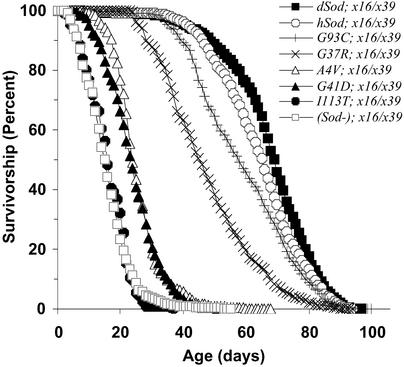
Survivorship curves of various Drosophila lines with FALS-associated Sod mutations. Pooled data are shown for each line type. The mean life spans of the replicate lines of each line type are shown in Table 1 (Experiment 2).
An important objective was to establish whether human wild-type Sod expression could reverse the life-span reduction observed in Sodx16/x39 flies. The majority of the hSOD; Sodx16/x39 lines expressed Cu-Zn SOD at only ≈5–10% of the wild-type level (Table 1, Fig. 1), and there was no compensatory rise in Mn SOD activity. However, six of these lines had life spans as long as the wild type (98% of the dSod; Sodx16/x39 mean, Fig. 2). Although one hSOD; Sodx16/x39 line had a shorter life span than the dSod; Sodx16/x39 flies, this line had only 2% of wild-type SOD activity. The level of SOD activity present in wild-type flies is thus in enormous excess of the amount required to sustain a normal life span.
FALS Sod Expression Confers Incomplete Rescue of Life Span.
A key prediction of this study was that FALS mutant Sod transgenes alone would not fully restore the wild-type life span when placed in a Sodx16/x39 background. It was reasoned that the decrease in life span might be due either to a loss or a gain of function, depending on the levels of SOD activity and results of subsequent crosses combining the mutant and hSOD alleles in the Sodx16/x39 background. In fact, the 18 mutant lines examined in this study exhibited a variety of phenotypes, mostly involving loss of SOD activity and partial rescue or recovery of life span [the average life span of (Sod−); Sodx16/x39 flies was defined as 0% and dSod; Sodx16/x39 flies as 100% recovery].
The most common Sod mutation in human FALS patients, SodA4V, was examined in six independent lines. No SOD activity was detectable in any of these SodA4V; Sodx16/x39 lines, but they had an average 17–19% recovery of life span in two independent experiments, and all six lines lived longer than any (Sod−); Sodx16/x39 line. Possibly some SOD activity exists in these mutants, accounting for the partial rescue of life span, but it could not be detected due to a decrease in the stability of the enzyme.
The SOD activity of the SodG41D-1; Sodx16/x39 mutant line was comparable to that of most hSod; Sodx16/x39 lines, 9% of wild type, but the electrophoretic mobility of SODG41D was increased, presumably due to its extra negative charge, and there was only 26% recovery of normal life span. Conversely, the electrophoretic mobility of SOD from SodG37R-1; Sodx16/x39 flies was impaired, consistent with its extra positive charge, resulting in the virtual comigration of SODG37R with Mn SOD (Fig. 1). Pretreatment with 2% SDS for 30 min, which eliminates Mn SOD but not Cu-Zn SOD activity (24), revealed the SODG37R-1 mutant activity unmistakably. Although this line had a nearly normal life span, two additional SodG37R; Sodx16/x39 lines (and SodG41D-2; Sodx16/x39) had shorter life spans and no detectable SOD activity. Thus, as has been observed in FALS families (25), the specific mutation was not a fully reliable predictor of longevity.
Whereas SODG93C and SODI113T had equivalent levels of activity in COS-1 cells (4), the corresponding alleles produced the most divergent phenotypes in Drosophila. The mildest effects on life span were observed among five mutant lines expressing the SodG93C allele, all of which had levels of SOD activity comparable to the hSod; Sodx16/x39 controls. The life span of SodG93C-2; Sodx16/x39 flies was essentially the same as in hSod; Sodx16/x39 controls, whereas the other four lines had incomplete or inconsistent recovery of life span. By contrast, SodI113T-1; Sodx16/x39 flies had no detectable SOD activity, and no recovery of life span in comparison with (Sod−); Sodx16/x39 controls. Thus, this line fulfilled the predictions of the loss-of-function hypothesis for FALS SOD. There was also no SOD activity in SodI113T-2; Sodx16/x39 flies, and a large majority of these flies died within 2 days post-eclosion. This line had an even shorter life span than the Sodx16/x39 controls, perhaps due to loss of activity coupled with a deleterious insertional position effect of the SodI113T transgene.
Measurement of rates of oxygen consumption in selected lines excluded the possibility that compensatory alterations in metabolic rate were responsible for the FALS Sod-associated variations in life span.
Life-Span Effects of FALS Sod Are Recessive.
To clarify whether the effects of FALS Sod on life span were dominant or recessive, and thus whether they were likely due to gain of function or to loss of SOD activity, the human mutant and wild-type Sod alleles were placed together in a y w; Sodx16/x39 background. A total of 24 y w; FALS Sod/hSod; Sodx16/x39 and four y w; hSod/hSod; Sodx16/x39 combinations were generated (n = 100 male progeny per cross). Their median life spans were 70 ± 7 and 70 ± 3 days, respectively. None of the individual mutations was associated with life spans consistently below this average. Thus, the incomplete rescue of life span by FALS Sod alleles in the Sodx16/x39 background was 100% recessive. Other crosses, substituting dSod for hSod in the Sodx16/x39 background, produced essentially the same result.
Oxidative Stress.
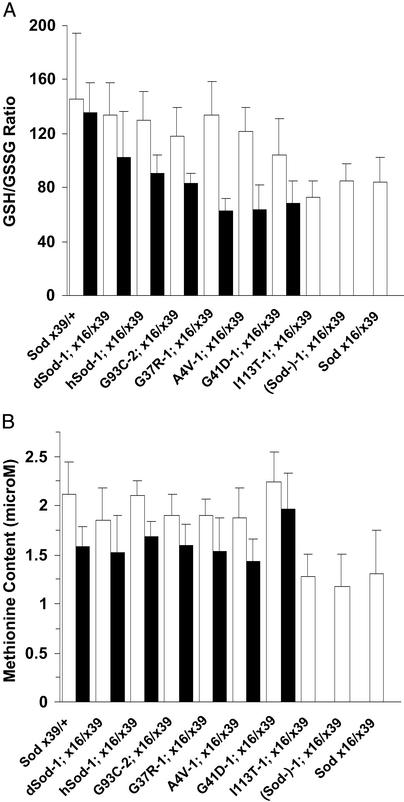
The effects of the mutant Sod alleles on oxidative stress, in the absence of wild-type Sod, were assessed by measuring the balance between reduced and oxidized glutathione (GSH/GSSG) and the level of free Met, which is highly susceptible to oxidation (26). Both of these markers of oxidative stress reflected more pro-oxidizing conditions in older flies and (Sod−); Sodx16/x39 flies, and a rapid shift from normal to pro-oxidizing conditions during mid-adult life in FALS Sod mutants (Fig. 3).
Figure 3.
Markers of oxidative stress in FALS Sod transgenic lines: GSH/GSSG (A) and methionine (B). Results are mean ± SD of 3–14 independent homogenates for each group and age. Open bars: 11–18 days. Filled bars: 45–50 days, except for groups SodA4V-1; Sodx16/x39 and SodG41D-1; Sodx16/x39 (20–24 days).
Among young adults (ages 11–18 days), the GSH content was the same in all lines examined except SodI113T-1; Sodx16/x39, (Sod−)-1; Sodx16/x39, and Sodx16/x39, in which it was 20–35% lower than in all other lines (P < 0.0005). There were only marginal differences in GSSG content. After 1 additional week (ages 20–24 days), the GSH content decreased by 10–20% in SodA4V-1; Sodx16/x39 and SodG41D-1; Sodx16/x39 flies (P < 0.05), whereas GSSG increased 35–55% (P < 0.005). Similarly, in old SodG37R-1; Sodx16/x39 flies (45–50 days), the GSH content decreased 8% (P < 0.01), whereas GSSG rose 87% (P < 0.0001). Consistent with earlier reports (20), there was no age-associated decrease in GSH content of Sodx39/+, dSod-1; Sodx16/x39, or hSod-1; Sodx16/x39 flies, or in the SodG93C-2; Sodx16/x39 mutant with full recovery of wild-type life span. The GSSG contents of these lines rose 30–40% by ages 45–50 days, except in the Sodx39/+ flies. Thus, the GSH/GSSG ratios (Fig. 3A) of Sod null and SodI113T-1; Sodx16/x39 flies were diminished due to lower GSH content, whereas in three FALS Sod mutants, premature death was preceded by a significant rise in GSSG content.
The Met contents of the positive control lines, SodG93C-2; Sodx16/x39 and SodG37R-1; Sodx16/x39 mutants decreased 15–25% with age (P < 0.0005) (Fig. 3B). Among young adults, Met levels were 30–40% lower in SodI113T-1; Sodx16/x39, (Sod–)-1; Sodx16/x39 and Sodx16/x39 flies than in the other groups (P < 0.05). Similar to the GSH content, Met was initially present in SodA4V-1; Sodx16/x39 flies at levels comparable to those of dSOD; Sodx16/x39 controls, but it declined 23% within 1 week before the onset of mortality. There was also a smaller decrement in SodG41D-1; Sodx16/x39 Met content. Altogether, the age- and genotype-dependent reductions in free Met content are consistent with the idea that protein-bound Met functions as an antioxidant, consuming oxidants that might otherwise damage critical functional sites within the protein molecule (27). By contrast, no strain-specific or age-dependent differences in cysteine content were observed.
Physiological Decline with Abrupt Onset in FALS Sod Drosophila.
The antioxidant status of many FALS Sod mutant lines suggested that the incomplete rescue of life span was not due to chronically elevated oxidative stress. Instead, the low levels of SOD activity were associated with an increase in oxidative stress of more abrupt onset. Accordingly, a series of tests was performed, to test the hypotheses that the FALS Sod flies with short life spans were physiologically normal in early adult life, and that their premature deaths were also preceded by a period of rapid physiological decline.
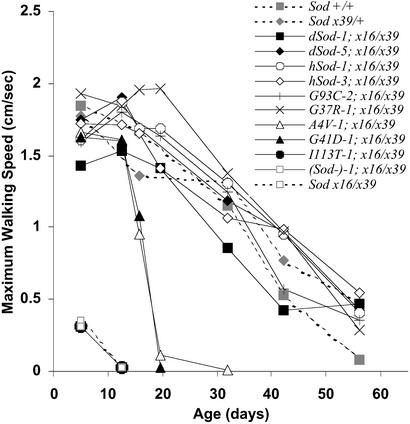
The first test measured the vertical walking speed of flies in negative geotaxis experiments (Fig. 4). Performance in this test was not only a function of speed and coordination, because a threshold of muscular strength was also required to gain a footing and make upward progress under the test conditions. As predicted by the loss-of-activity hypothesis, the performance of SodI113T-1; Sodx16/x39 and SOD null flies was dramatically impaired, even at the youngest age tested (5 days). The long-lived mutant and control lines performed robustly as young adults, but their speed of walking decreased continuously with increasing age. The most striking finding was that the SodA4V-1; Sodx16/x39 and SodG41D-1; Sodx16/x39 flies, which live less than half as long as the controls, initially matched the walking speed of the control lines and did not show a progressive age-related decrease in speed. Instead, these flies maintained the same motor ability as the controls until 12 days of age, after which the maximum height they could attain declined rapidly to near zero by 19 days, approximately the age at which increased mortality rates were first observed.
Figure 4.
Negative geotaxis. The vertical walking speed was measured in 12-sec intervals, three times for each fly, and the maximum height attained was expressed as speed per second. Each point represents the average of 15–45 flies.
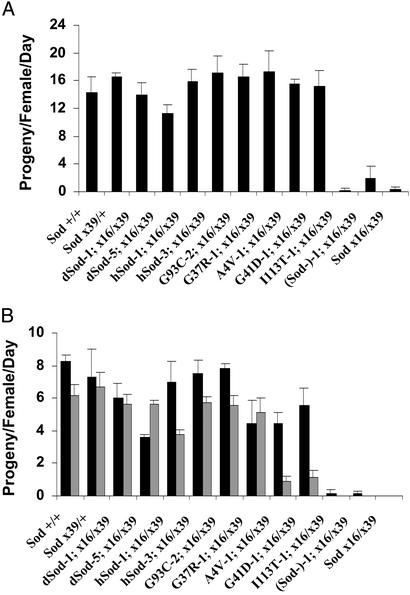
Similar to male geotactic activity, the female fertility of SodA4V-1; Sodx16/x39 and SodG41D-1; Sodx16/x39 flies was initially unimpaired, compared with multiple control lines, but subsequently underwent a sharp decline (Fig. 5). However, the period in which normal physiological function was maintained was shorter and less striking in the fertility experiments. The fertility of female flies was almost totally abolished in the Sodx16/x39, (Sod−)-1; Sodx16/x39 and SodI113T-1; Sodx16/x39 lines, but neither the Sod null nor the FALS Sod genotypes had any detrimental effect on male fertility.
Figure 5.
Female fertility. Virgin females (five per vial) were outcrossed with five outbred wild-type males 2–3 days after collection. The numbers of adult progeny were determined after full eclosion and reported as mean ± SD of three replicate crosses. In a second experiment, similar results were obtained, except that fertility diminished less rapidly with increasing age. (A) First 24 h after mating. (B) Average fertility, days 2–4 (black bars) and days 5–14 (gray bars) after mating.
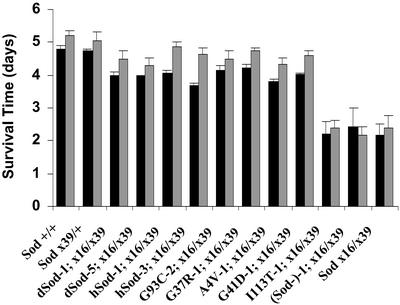
Finally, in a test of resistance to experimental oxidative stress, flies aged 9–10 days were exposed to hyperoxia (100% oxygen), which accelerates the production of superoxide anion radicals (28), the substrate for SOD. If the SodA4V-1; Sodx16/x39 and SodG41D-1; Sodx16/x39 animals were approaching a threshold of impaired physiological performance in this age range, due to diminished SOD activity, then this oxidative challenge should expose their latent weakness, resulting in decreased survival times. In fact, these flies displayed resistance to hyperoxia equivalent to the positive control lines (Fig. 6), whereas SOD null flies had decreased survival times. This observation reinforced the conclusion that these FALS Sod flies were phenotypically normal before the onset of FALS-linked pathology.
Figure 6.
Resistance to experimental oxidative stress. Flies were housed in three groups of 10 and exposed continuously to 100% oxygen beginning at 9–10 days of age. Mortality was scored once or twice daily. Results shown are mean ± SD for two independent experiments, represented by black and gray bars.
Discussion
The main objective of this study was to determine whether the expression of FALS Sod alleles produces similar effects in species as diverse as humans and flies. Expression of human wild-type SOD at very low levels was sufficient to rescue the life-span reduction, increased oxidative stress, and impaired physiological function associated with a Drosophila Sod null background. However, the introduction of FALS Sod alleles only partly reversed these effects. In the mutant flies, oxidative stress increased before the onset of mortality, but it was not elevated throughout adult life. Similarly, physiological function was not initially impaired, nor did it decline at a continuous, accelerated rate throughout adult life. Rather, the results are consistent with pathological changes of abrupt onset leading to premature death. This premature death was recessive in the presence of either a human or a Drosophila wild-type allele.
Although there is some opposing evidence in the case of SOD (12), the majority of studies indicate that enzymatic antioxidants are present in excess in Drosophila, and large increases or decreases in their activities have no appreciable effect on longevity (11, 29, 30). The life spans of the hSod; Sodx16/x39 flies reinforce this conclusion, because activity levels as low as 4% of wild type were associated with full rescue of life span. Given the finding that human SOD targeted to motor neurons actually increases life span in Drosophila (13), it is conceivable that human SOD scavenges free radicals more efficiently than fly SOD. Alternatively, the results suggest that SOD activity does not determine longevity in Drosophila, unless it falls below 5% of the wild-type level or is expressed with an abnormal spatial distribution. Thus, the life spans of the control strains in this study should help to resolve an ongoing debate about the relationship between levels of SOD activity and the aging process in Drosophila.
Given the low level of SOD activity required to maintain the life span of Drosophila, and the low levels actually observed in the hSod and FALS Sod mutant lines, the presence of a wild-type allele would be predicted to mask recessive effects of the mutant alleles, whereas dominant effects should be apparent either in the presence or the absence of the wild-type allele. Because the combination of either hSod or dSod with the FALS Sod alleles resulted in complete recovery of wild-type life span, the reduction of SOD activity below a critical threshold (≈5% of wild type) is the most likely explanation for the observed phenotypes.
It is striking that activity levels below this threshold, and even below the detection limits of the assays used in this study, confer partial rescue of life span, and that physiological function is essentially the same as in wild-type flies throughout much of the abbreviated adult phase. These results suggest that insufficient levels of SOD activity do not cause chronic oxidative stress in the flies, possibly because other radical scavengers compensate temporarily for the low level of SOD activity, after which their premature depletion leads to a catastrophic increase in oxidative stress. The ensuing steep decline in motor and other physiological capabilities, leading to death, has at least a superficial resemblance to human FALS. However, the results of this study, in conjunction with earlier findings from FALS Sod expression restricted to motor neurons (13), suggest that the introduction of FALS mutant Sod alleles in Drosophila does not result in a dominant gain of function, as is observed in human patients.
Acknowledgments
This work was supported by grants from the ALS Association and the National Institute on Aging–National Institutes of Health (RO1 AG7657). The human SOD cDNAs were generous gifts of R. H. Brown (Massachusetts General Hospital, Boston) and B. A. Hosler, and D. R. Borchelt (The Johns Hopkins University School of Medicine, Baltimore). Assistance with the experiments was provided by J. G. Hubbard, L. Lam, M. Orona, E. C. Orr, I. Rebrin, and S. Tran.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- FALS
familial ALS
- SOD
Cu-Zn superoxide dismutase
- GSSG
oxidized glutathione
- GSH
reduced glutathione
References
- 1.Brown R H., Jr Cell. 1995;80:687–692. doi: 10.1016/0092-8674(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 2.Rowland L P, Shneider N A. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 3.Deng H-X, Hentati A, Tainer J A, Iqbal Z, Cayabyab A, Hung W-Y, Getzoff E D, Hu P, Herzfeldt B, Roos R P, et al. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 4.Borchelt D R, Lee M K, Slunt H S, Guarnieri M, Xu Z-S, Wong P C, Brown R H, Jr, Price D L, Sisodia S S, Cleveland D W. Proc Natl Acad Sci USA. 1994;91:8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yim M B, Kang J-H, Yim H-S, Kwak H-S, Chock P B, Stadtman E R. Proc Natl Acad Sci USA. 1996;93:5709–5714. doi: 10.1073/pnas.93.12.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crow J P, Sampson J B, Zhuang Y, Thompson J A, Beckman J S. J Neurochem. 1997;69:1936–1944. doi: 10.1046/j.1471-4159.1997.69051936.x. [DOI] [PubMed] [Google Scholar]
- 7.Gurney M E, Pu H, Chiu A Y, Dal Canto M C, Polchow C Y, Alexander D D, Caliendo J, Hentati A, Kwon Y W, Deng H-X, et al. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 8.Bruijn L I, Houseweart M K, Kato S, Anderson K L, Anderson S D, Ohama E, Reaume A G, Scott R W, Cleveland D W. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 9.Jung C, Rong Y, Doctrow S, Baudry M, Malfroy B, Xu Z. Neurosci Lett. 2001;304:157–160. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- 10.Phillips J P, Tainer J A, Getzoff E D, Boulianne G L, Kirby K, Hilliker A J. Proc Natl Acad Sci USA. 1995;92:8574–8578. doi: 10.1073/pnas.92.19.8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orr W C, Sohal R S. Arch Biochem Biophys. 1993;301:34–40. doi: 10.1006/abbi.1993.1111. [DOI] [PubMed] [Google Scholar]
- 12.Parkes T L, Elia A J, Dickinson D, Hilliker A J, Phillips J P, Boulianne G L. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 13.Elia A J, Parkes T L, Kirby K, St. George-Hyslop P, Boulianne G L, Phillips J P, Hilliker A J. Free Radical Biol Med. 1999;26:1332–1338. doi: 10.1016/s0891-5849(98)00333-5. [DOI] [PubMed] [Google Scholar]
- 14.Klichko V I, Radyuk S N, Orr W C. Neurobiol Aging. 1999;20:537–543. doi: 10.1016/s0197-4580(99)00090-1. [DOI] [PubMed] [Google Scholar]
- 15.Pramatarova A, Laganière J, Roussel J, Brisebois K, Rouleau G A. J Neurosci. 2001;21:3369–3374. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feany M B, Bender W W. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 17.Thummel C S, Boulet A M, Lipshitz H D. Gene. 1988;74:445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- 18.Mockett R J, Orr W C, Sohal R S. Methods Enzymol. 2002;349:213–220. doi: 10.1016/s0076-6879(02)49336-6. [DOI] [PubMed] [Google Scholar]
- 19.Beauchamp C, Fridovich I. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 20.Mockett R J, Sohal R S, Orr W C. FASEB J. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- 21.Spitz D R, Oberley L W. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 22.Tyler R H, Brar H, Singh M, Latorre A, Graves J L, Mueller L D, Rose M R, Ayala F J. Genetica. 1993;91:143–149. doi: 10.1007/BF01435994. [DOI] [PubMed] [Google Scholar]
- 23.Phillips J P, Campbell S D, Michaud D, Charbonneau M, Hilliker A J. Proc Natl Acad Sci USA. 1989;86:2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geller B L, Winge D R. Anal Biochem. 1983;128:86–92. doi: 10.1016/0003-2697(83)90348-2. [DOI] [PubMed] [Google Scholar]
- 25.de Belleroche J, Orrell R, King A. J Med Genet. 1995;32:841–847. doi: 10.1136/jmg.32.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskovitz J, Berlett B S, Poston J M, Stadtman E R. Proc Natl Acad Sci USA. 1997;94:9585–9589. doi: 10.1073/pnas.94.18.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine R L, Mosoni L, Berlett B S, Stadtman E R. Proc Natl Acad Sci USA. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turrens J F, Freeman B A, Levitt J G, Crapo J D. Arch Biochem Biophys. 1982;217:401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- 29.Reveillaud I, Niedzwiecki A, Bensch K G, Fleming J E. Mol Cell Biol. 1991;11:632–640. doi: 10.1128/mcb.11.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orr W C, Arnold L A, Sohal R S. Mech Ageing Dev. 1992;63:287–296. doi: 10.1016/0047-6374(92)90006-y. [DOI] [PubMed] [Google Scholar]