Abstract
(1) A direct experimental method has been used to test whether IgM immunological memory is carried by antibody-forming cells or by cells not releasing detectable antibody.
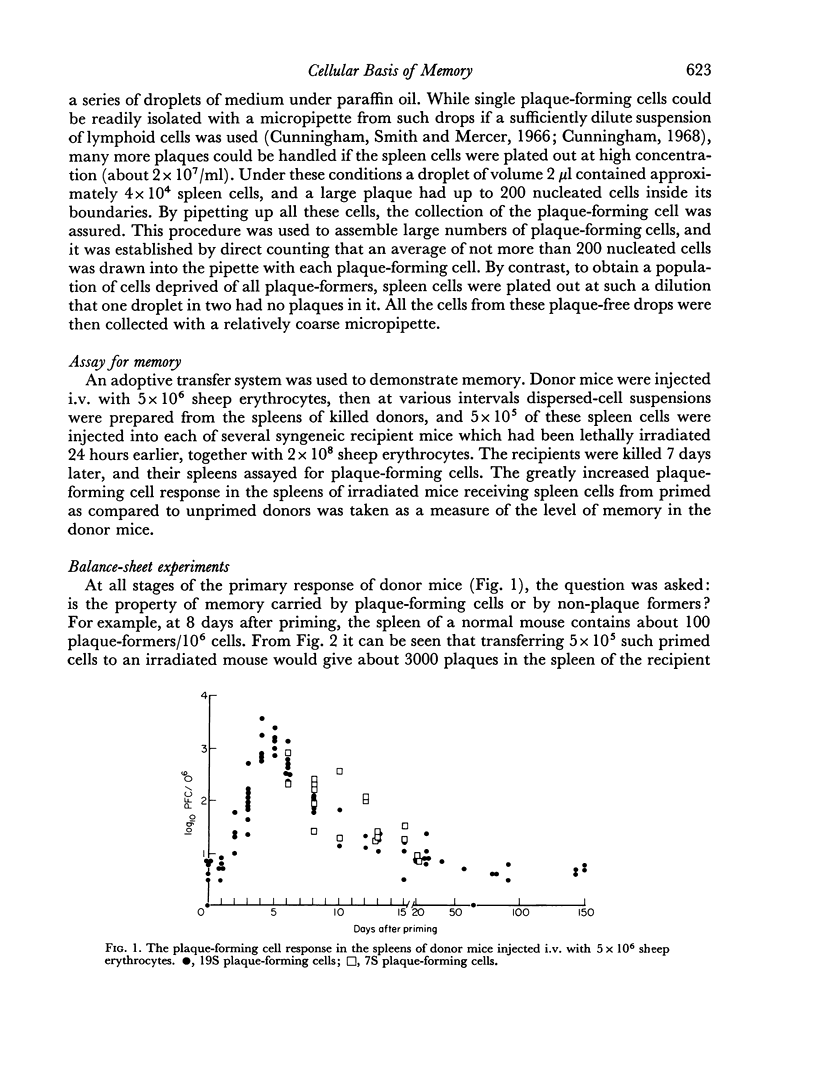
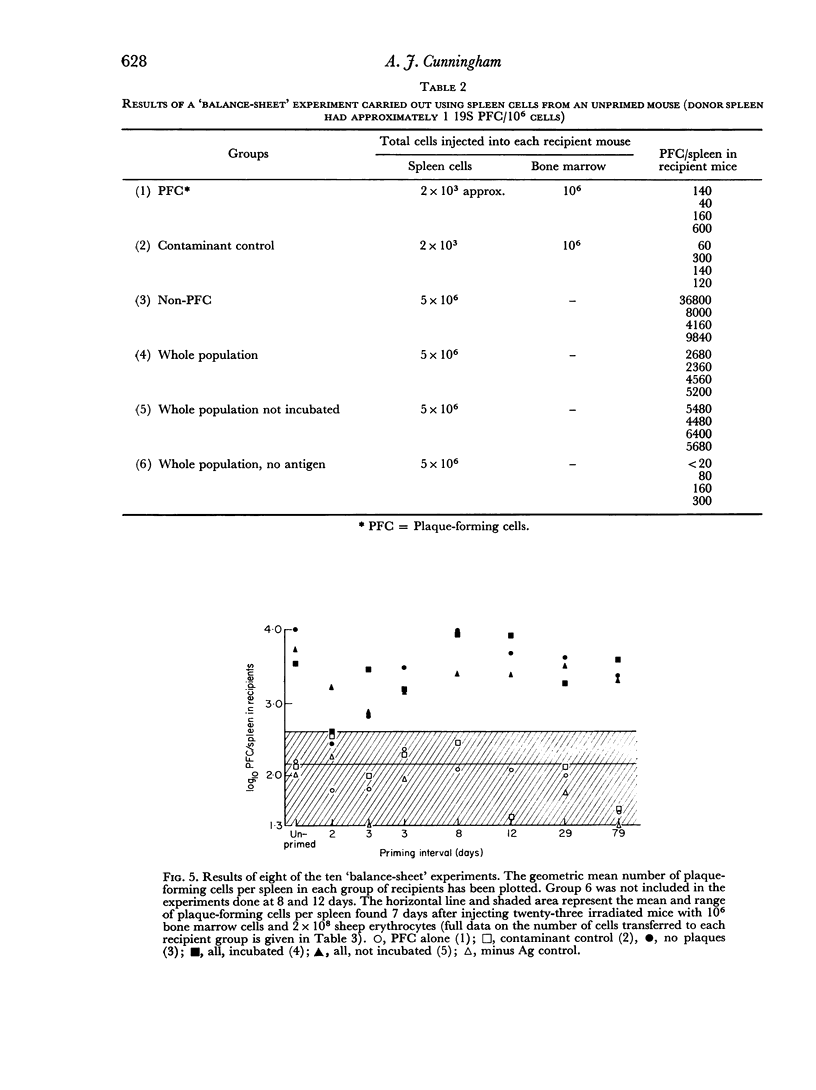
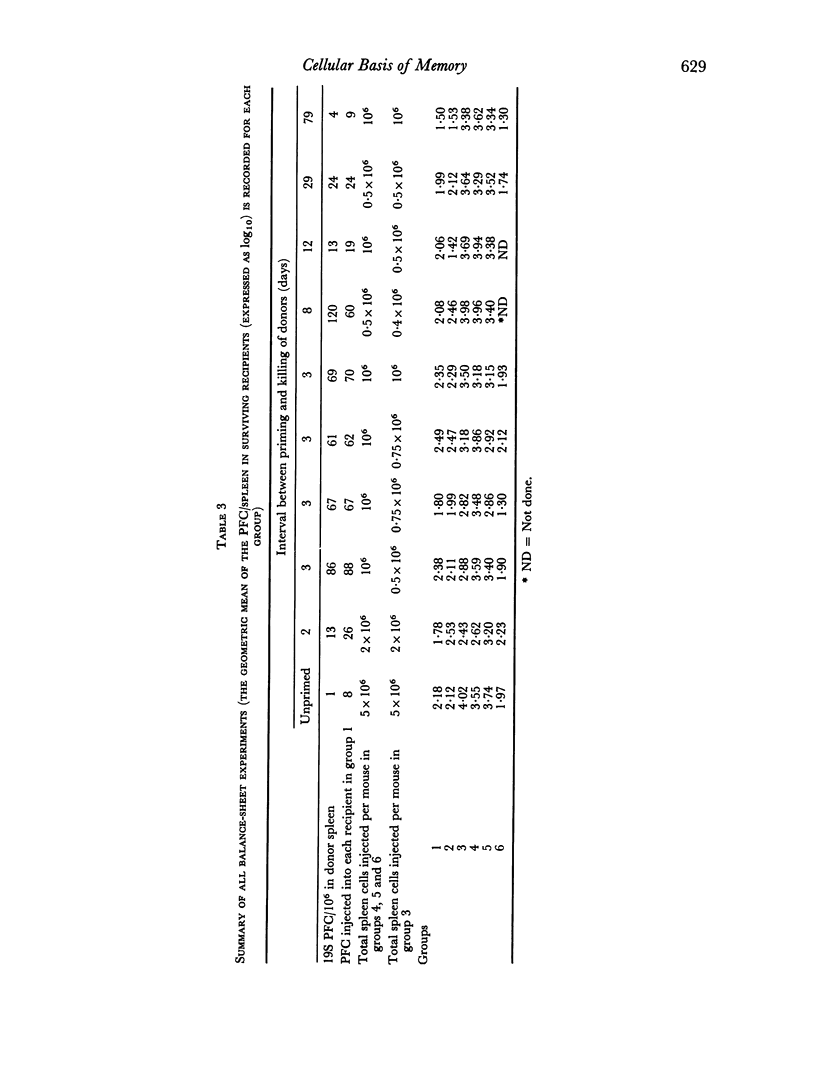
(2) An adoptive transfer system was used to demonstrate memory. Donor mice were injected intravenously (i.v.) with sheep erythrocytes, and at intervals thereafter, dispersed cells from their spleens were transferred to lethally irradiated recipient mice, together with more antigen. The greatly increased plaque-forming cell responses in the spleens of irradiated mice receiving primed as compared to unprimed donor cells was taken as a measure of the level of IgM memory in the donor mice.
(3) Suspensions of donor spleen cells were divided mechanically (by micromanipulation) into: (a) a plaque-forming cell fraction, and (b) a fraction totally free of plaque-forming cells. Each fraction was injected, with antigen, into an irradiated recipient mouse, and the response of these mice was compared with that of a third animal which received unfractionated cells. It was consistently found that irradiated mice receiving plaque-free populations of cells responded as well as the controls, while no response was observed in mice receiving donor plaque-forming cells alone. A similar approach showed that the antigen responsiveness of spleen cells from unprimed mice is independent of their content of `background' plaque-forming cells. It is concluded that IgM immunological memory is carried by cells which are not producing plaques, and reasons are discussed for supposing that memory cells and antibody-forming cells belong to separate cell lineages.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Wilkes B. Immunological tolerance induced by cyclophosphamide assayed by plaque spleen cell method. Nature. 1967 Feb 4;213(5075):498–499. doi: 10.1038/213498a0. [DOI] [PubMed] [Google Scholar]
- Celada F. Quantitative studies of the adoptive immunological memory in mice. II. Linear transmission of cellular memory. J Exp Med. 1967 Feb 1;125(2):199–211. doi: 10.1084/jem.125.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Smith J. B., Mercer E. H. Antibody formation by single cells from lymph nodes and efferent lymph of sheep. J Exp Med. 1966 Oct 1;124(4):701–714. doi: 10.1084/jem.124.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J. The morphology of antibody-forming cells in the mouse. Aust J Exp Biol Med Sci. 1968 Apr;46(2):141–153. doi: 10.1038/icb.1968.12. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- HELMREICH E., KERN M., EISEN H. N. The secretion of antibody by isolated lymph node cells. J Biol Chem. 1961 Feb;236:464–473. [PubMed] [Google Scholar]
- MOLLER G., WIGZELL H. ANTIBODY SYNTHESIS AT THE CELLULAR LEVEL. ANTIBODY-INDUCED SUPPRESSION OF 19S AND 7S ANTIBODY RESPONSE. J Exp Med. 1965 Jun 1;121:969–989. doi: 10.1084/jem.121.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Immunological activity of thymus and thoracic-duct lymphocytes. Proc Natl Acad Sci U S A. 1968 Jan;59(1):296–303. doi: 10.1073/pnas.59.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G. Regulation of cellular antibody synthesis. Cellular 7S production and longevity of 7S antigen-sensitive cells in the absence of antibody feedback. J Exp Med. 1968 Feb 1;127(2):291–306. doi: 10.1084/jem.127.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettesheim P., Makinodan T., Williams M. L. Regenerative potential of immunocompetent cells. I. Lack of recovery of secondary antibody-forming potential after x-irradiation. J Immunol. 1967 Jul;99(1):150–157. [PubMed] [Google Scholar]
- Nossal G. J., Austin C. M., Ada G. L. Antigens in immunity. VII. Analysis of immunological memory. Immunology. 1965 Oct;9(4):333–348. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. The mechanism of action of antigen. Australas Ann Med. 1965 Nov;14(4):321–328. doi: 10.1111/imj.1965.14.4.321. [DOI] [PubMed] [Google Scholar]
- Sercarz E. E., Byers V. S. The X-Y-Z scheme of immunocyte maturation. 3. Early IgM memory and the nature of the memory cell. J Immunol. 1967 Apr;98(4):836–843. [PubMed] [Google Scholar]
- Sterzl J. Immunological tolerance as the result of terminal differentiation of immunologically competent cells. Nature. 1966 Jan 22;209(5021):416–417. doi: 10.1038/209416a0. [DOI] [PubMed] [Google Scholar]
- Sterzl J., Ríha I. Detection of cells producing 7S antibodies by the plaque technique. Nature. 1965 Nov 27;208(5013):858–859. doi: 10.1038/208858a0. [DOI] [PubMed] [Google Scholar]
- Szenberg A., Cunningham A. J. DNA synthesis in the development of antibody-forming cells during the early stages of the immune response. Nature. 1968 Feb 24;217(5130):747–748. doi: 10.1038/217747a0. [DOI] [PubMed] [Google Scholar]
- Vischer T. L., Stastny P., Ziff M. Development of immunological memory during the primary immune response. Nature. 1967 Mar 4;213(5079):923–925. doi: 10.1038/213923b0. [DOI] [PubMed] [Google Scholar]
- Wigzell H. The rise and fall of 19S immunological memory against sheep red cells in the mouse. Ann Med Exp Biol Fenn. 1966;44(2):209–215. [PubMed] [Google Scholar]


