Abstract
Microarrays comprise an efficient approach to discovering large numbers of differentially expressed mRNA transcripts in the CNS resulting from changes in hormonal milieu. We used high-density oligonucleotide microarrays to examine the short- and long-term actions of estradiol (E2) on the transcriptomes from the medial basal hypothalamus and other brain regions of E2-treated (10 μg) adult female mice. Our results have revealed several unanticipated gene regulations. Most striking is lipocalin prostaglandin D2 synthase (L-PGDS), which catalyzes the conversion of prostaglandin (PG) H2 to PGD2, a neuromodulator involved in a variety of functions, including sleep, pain, and odor responses. In situ hybridization revealed significant increases in L-PGDS expression in the arcuate and ventromedial nucleus of the medial basal hypothalamus compared with vehicle controls. The magnitude of these changes is ≈2-fold and suggests a modulatory role for PGD2 in E2-controlled neuroendocrine secretions and behaviors. Surprisingly, L-PGDS gene expression is reduced 2-fold after E2 treatment in the ventrolateral preoptic area (VLPO), the suspected site of action for the sleep-promoting effects of PGD2. Finally, whereas L-PGDS has been reported to be expressed primarily in oligodendrocytes of the adult rodent brain, we demonstrate, immunocytochemically, that L-PGDS is also expressed in a population of VLPO neurons. Thus, our data suggest the intriguing possibility that E2 modulation of L-PGDS plays a role in the regulation of sleep–wake states through hitherto unknown mechanisms in VLPO neurons and through hormone-dependent neuronal-glial cooperation.
Keywords: microarrays‖preoptic area‖oligodendrocytes‖estrogens‖arousal
Gonadal estrogens have wide-ranging effects in the central nervous system (CNS) of most adult mammals, including rodents and humans. These effects include but are not limited to the regulation of endocrine secretions from the anterior pituitary (1–4), sex-specific behaviors in rodents (5, 6), learning and memory (7–9), and neuroprotection (10–12). Estrogens are also known to regulate the expression of a number of different genes in the CNS (6). Despite this knowledge, we lack a complete understanding of estrogen-targeted genes in the CNS and how changes in their transcript levels may contribute to the myriad of neurobiological functions affected by estrogens.
Microarray technology is a powerful method for assessing the changes simultaneously in thousands of gene transcripts in neuroendocrine systems (reviewed in ref. 13). It can reveal the orchestration of genomic changes taking place within the cells of the CNS and can lead to unanticipated discoveries of genomic alterations. We have been using high-density oligonucleotide arrays to elucidate the short- and long-term actions of estrogens on the transcriptomes from the medial basal hypothalamus (MBH) and other brain regions of female adult mice. Because the functional consequences of estrogens acting at the level of the MBH are well documented (see above), this region is an excellent model for beginning to address hitherto unknown molecular mechanisms. Arrays hybridized with RNA from the MBH of estradiol (E2)-treated and vehicle-treated adult female mice have revealed several surprising gene candidates that may be regulated by E2. One of the more interesting genes is lipocalin-prostaglandin D2 synthase (L-PGDS), a nonneuronal enzyme that catalyzes the conversion of prostaglandin (PG) H2 to PGD2, which is involved in a variety of functions, including sedation and sleep (14). By using microarray data from the MBH as a springboard for uncovering previously uncharacterized gene regulation by E2, we demonstrate that E2 can induce or repress L-PGDS gene expression in different regions of the brain in a manner suggesting that steroids may also be capable of modulating sleep–wake patterns.
Materials and Methods
Animals and Microarray Probe Preparation.
Forty ovariectomized female adult Swiss–Webster mice (Taconic Farms) were randomly placed in control (i.p. injection of 0.05 ml of DMSO, n = 20) or E2-treated (10 μg 17β-E2 in DMSO, n = 20) groups. The mice were housed according to their treatment groups and allowed food and water ad libitum. After treatment, the mice from both groups were killed at either 2 h (n = 10 for both Veh and E2) or 24 h (n = 10 for both Veh and E2). The MBH was dissected after the delineation in the Franklin and Paxinos mouse brain atlas (15). The brains were removed, immediately placed in an adult mouse brain matrix (Ted Pella, Inc., Redding, CA), chilled on ice, and sectioned at 2-mm intervals. The MBH was rapidly microdissected from the 2-mm coronal slice, which was 1-mm caudal from the optic chiasm. From that identified slice, a trapezoid containing the MBH was dissected by making two diagonal cuts from the dorsal tip of the third ventricle to the base of the brain and a third cut at the dorsal tip of the third ventricle and parallel to the base of the brain. The tissue was placed into ice-cold RNAlater (Ambion, Austin, TX) and stored at −80°C. The MBH from each group was pooled according to treatment and time of death; at least 5 μg of poly(a)+ RNA was purified from each group and converted to ≈20 μg of biotinylated cRNA, according to the Affymetrix (Santa Clara, CA) protocol for probe preparation. All tissue was treated identically.
Affymetrix Microarray Probe Hybridization and Data Analysis.
Mu11kA and Mu11kB, high-density oligonucleotide microarrays (also known as GeneChips; ref. 16) together representing 11,566 mouse genes or EST sequences, were purchased from Affymetrix. The biotinylated cRNA from each treatment group was hybridized to its own GeneChip according to the Affymetrix protocol. The hybridization and scanning of the GeneChips were carried out at Wyeth Ayerst Laboratories (Marietta, PA). The purpose of our microarray screens was to identify candidate genes targeted by E2 and not to provide an accurate measurement of the changes in transcript quantity in the presence of E2. Therefore, a single set of GeneChips (Mu11kA and Mu11kB) was used for each probe. Suspected candidate genes were validated with in situ hybridization (see below).
The data obtained are reported as the “average differences” of each gene present on the GeneChip. Briefly, each gene or EST is represented by at least 16 nonoverlapping 25-mer nucleotide sequences termed “perfect-match probes.” Each perfect-match probe was paired with a “mismatch” or control probe containing a single-base substitution. Thus, the average difference assigned to each gene or EST was the average of the signals from the specific perfect-match probes minus the average signal from the corresponding mismatch probes. The data were normalized by using MICROARRAY SUITE V.4.0 software (Affymetrix) to correct for varying background noise and small differences in the amount of each cRNA probe applied to the microarrays. Comparisons were made between vehicle-treated and E2-treated tissue, with vehicle being the baseline measurement. Results were reported as the “fold change” over the baseline. A gene in the E2-treated animals was considered induced or repressed if (i) the normalized average difference value was above the background value of the GeneChip and (ii) the fold change exceeded 2.5 in either direction (i.e., induced or repressed).
L-PGDS Probe Preparation.
Total RNA isolated from an adult Swiss–Webster mouse brain according to the Trizol reagent (GIBCO/BRL) protocol was transcribed into single-stranded cDNA by using SuperScript Choice (GIBCO/BRL). L-PGDS-specific primer pairs were designed based on the mouse cDNA sequence from GenBank with the aid of MACVECTOR V.7.1.1SL (Accelrys, San Diego). The primer sequences were: forward, 5′-TGCTTGCTTTGTCCACATTGC-3′; reverse, 5′-CTTGGTGCCTCTGCTGAATAGC-3′. PCR amplification of the cDNA yielded the expected 470-bp fragment, and the sequence was confirmed at the DNA Sequencing Resource Center (The Rockefeller University, New York). The amplicon was subsequently used as a template in the synthesis of probes for Northern blotting and in situ hybridization.
Northern Blotting.
Four brain regions were chosen for analysis of L-PGDS expression in ovariectomized female adult Swiss–Webster mice: cortex, olfactory bulb, preoptic area, and the MBH. The mice (n = 4 per group and two separate experiments were performed) were treated as described and killed 24 h after treatment. Tissue from the four brain regions was pooled according to region and treatment, and then homogenized in Trizol reagent according to the manufacturer's specifications, and 10 μg of total RNA was resolved by agarose (1.2%)/formaldehyde (6.5%)/Mops gel electrophoresis (17). The separated total RNA was transferred to Hybond nitrocellulose (Amersham Pharmacia) and fixed by baking for 2 h at 80°C. The blots were probed with a 32P-labeled L-PGDS fragment by using ExpressHyb (CLONTECH) solution, according to the manufacturer's protocol. After the final wash, the blots were exposed to Biomax film (Kodak) for 30 min, and the film was developed according to the manufacturer's specifications. To test for discrepancies in the amount of total RNA loaded per lane, the nitrocellulose membranes were then stripped and reprobed with a 32P-labeled PCR fragment complementary to the 18S subunit of ribosomal RNA. The films were digitized with an Epson scanner, and densitometry of the bands was performed by using the NIH IMAGE gel-analysis program. A density step wage (Kodak) was used to create a standard curve, and all measurements fell within the linear portion of that curve. Statistical analysis was performed with a two-way ANOVA (region × treatment) followed by a Neuman–Keuls post hoc test.
In Situ Hybridization.
Fourteen ovariectomized female, adult Swiss–Webster mice were treated as described (n = 7 per group) and killed 24 h after treatment. The brains were removed, immediately frozen with dry ice, and stored at −80°C until sectioned. The brains were cut into 12-μm-thick sections with a Bright cryostat and directly mounted onto electrostatically charged glass microscope slides. Sections were processed and hybridized with a 35S-labeled riboprobe to L-PGDS mRNA, according to the protocol outlined in ref. 18. The template for the L-PGDS probe was constructed by ligating a T7 promoter site directly to the PCR fragment with the aid of the Lig'nScribe kit (Ambion). This procedure was used for both the antisense and sense templates. The Maxiscript (Ambion) reagents and protocol were used to label the antisense and sense probes with 35S, and the labeled probes were hybridized to the tissue at 60°C. After the hybridization, the slides were washed, treated with Rnase A, dehydrated, and subjected to autoradiography for 3 days. Upon confirmation of a specific signal, the slides were exposed to liquid emulsion (Kodak NTB2) for 1 week and developed with standard photographic techniques.
Quantification of Reduced Silver Grains.
Reduced silver grains localized over cells were quantified with the aid of the MCID image analysis system (Imaging Research, St. Catherine's, ON, Canada). The distribution of grains was consistent with β emissions from the 35S isotope. In short, a “target size” of a single grain was established and used to determine “estimated counts” of reduced silver grains, proportional to pixel counts, over counterstained cells. Based on background counts, only cells with >20 grains were considered for analysis. For each animal, the values obtained represented the average of measurements taken from two to three sections per slide and two slides per animal. Statistical analysis was performed with a two-way ANOVA (region × treatment) followed by a Neuman–Keuls post hoc test.
Immunocytochemistry.
Paraffin-embedded brain sections (4 μm) from adult female mice (n = 4) were deparaffinized according to Schwanzel-Fukuda et al. (19), and immunoperoxidase staining was performed as reported with a 1:1,000 dilution of goat polyclonal L-PGDS primary antibody (Santa Cruz Biotechnology) in 1% normal rabbit serum (NRS) in PBS by the use of an avidin–biotin–horseradish peroxidase complex method (Vectastain ABC, Elite Kit, Vector Laboratories) according to standard protocols (20). As a control, the working dilution of the L-PGDS primary antibody was incubated with a peptide sequence homologous to L-PGDS. Tissue sections were treated with these preabsorbed antibodies as described above. For double immunostaining, a 1:30,000 dilution of mouse antineuronal nuclei antibody (NeuN, Chemicon) was used to identify neurons. The avidin–biotin–horseradish peroxidase complex was visualized with cobalt chloride- (0.025%) and ammonium nickel (II) sulfate (0.02%)-enhanced 3,3′-diaminobenzidine tetrahydrochloride reaction.
Results
By using the microarray data, pairwise comparisons of the average difference values between E2 vs. vehicle controls at 2 and 24 h in the MBH identified several obvious off-diagonal points that represented a 2.5-fold change (Fig. 1). Sequences from the L-PGDS gene (GenBank accession no. AB006361) were present in our screens and demonstrated a 2.7- and 3.1-fold induction at 2 and 24 h, respectively. PGD2, the product of the enzymatic reaction catalyzed by L-PGDS, is recognized as a putative sleep-promoting substance in the CNS; hence, its regulation by E2 is of particular interest to us.
Figure 1.
Linear scatter-plots of E2-induced changes in transcript levels in the MBH of adult ovariectomized female mice at 2 h (A) and 24 h (B) measured by the Mu11kA and Mu11kB high-density oligonucleotide microarrays. Each point represents a gene on the microarray, with coordinates derived from the signal intensities obtained from the hybridization of the E2-treated and vehicle-treated target probes from the appropriate time points. The axes are log scales of the average signal intensities. (A) At 2 h, 11% of the total genes represented demonstrated a 2-fold increase in transcript levels, whereas 5.6% of the genes demonstrated a 2-fold decrease. (B) At 24 h, 16% of the total genes represented demonstrated a 2-fold increase in transcript levels, whereas 4.6% of the genes demonstrated a 2-fold decrease.
Measurement of L-PGDS Gene Expression by Northern Blot Analysis.
Northern blot analysis confirmed the microarray data. Radiolabeled cDNA probes for L-PGDS detected the appropriate size transcript (721 bp) in total RNA extracted from various brain regions of vehicle and E2-treated adult ovariectomized female mice (Fig. 2A). By using computerized densitometry, we detected significantly higher levels of L-PGDS transcripts in RNA extracted from the MBH of E2-treated mice compared with vehicle-treated animals (Fig. 2B; P < 0.05, ANOVA; F(1,24) = 29.7). When normalized to 18S ribosomal RNA transcripts, the mean optical density for L-PGDS bands from the MBH was increased 2-fold in E2-treated animals compared with control animals. Interestingly, in the preoptic region (POA), our analysis revealed a dramatic down-regulation of L-PGDS gene expression after E2 treatment (Fig. 2B; P < 0.001, ANOVA). When normalized as above, the mean optical density for L-PGDS bands from the POA was decreased by 9-fold in E2-treated animals compared with control animals. There were no significant changes in L-PGDS expression detected in the cortex or olfactory bulb. The optical densities of the transcripts for the 18S ribosomal subunit were not significantly different between the treatment groups or regions.
Figure 2.
L-PGDS transcript levels were determined by Northern blot analysis after 24 h of E2 treatment. (A) Representative electrophoretic lanes from the cortex (ctx), MBH, olfactory bulb (OB), and POA from vehicle-treated (V) or E2-treated (E) adult ovariectomized female mice. Total RNA (10 μg) was loaded onto each lane. The blots were probed with a 32P-labeled L-PGDS, then stripped and reprobed with a 32P-labeled PCR fragment complementary to 18S subunit of ribosomal RNA to test for discrepancies in the amount of total RNA loaded per lane. (B) When standardized to the 18S ribosomal RNA transcripts, the mean optical density for L-PGDS bands from the MBH was increased 2-fold in E2-treated animals compared with control animals. In the POA, the mean optical density for L-PGDS was dramatically decreased by 9-fold in E2-treated animals compared with control animals. Data are represented as a ratio of the optical density of the L-PGDS bands to the 18S ribosomal subunit bands (*, P < 0.05, ANOVA).
Determination of L-PGDS Cellular Expression by in Situ Hybridization.
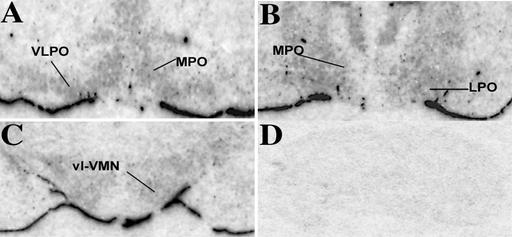
Because the MBH and POA contain many diverse brain nuclei and cell groups, the high spatial resolution technique of in situ hybridization was used to localize more precisely the cellular expression of L-PGDS mRNA. Analyses of autoradiographs indicated that L-PGDS mRNA was expressed in cells within both the parenchyma and leptomeninges (Fig. 3 A–C). Expression levels were greatest in the cortex, basal forebrain, medial and lateral POAs, and the MBH, whereas they were noticeably lower in the thalamus. No specific hybridization signal was observed in these same brain regions when the control, sense riboprobe was used (Fig. 3D). Brightfield microscopic evaluation of tissue sections after emulsion autoradiography for L-PGDS expression confirmed the presence of reduced silver grains over counterstained cells in the brain areas mentioned above, but not when hybridized with the sense probe. Based on the neuroanatomical descriptions in the Franklin and Paxinos mouse atlas (15), specific brain nuclei within the POA and MBH were identified for cellular analysis of reduced silver grains. In the rostral POA sections, the medial preoptic area (MPO) and the ventrolateral preoptic area (VLPO) were designated for analysis (Fig. 3A). In the caudal portion of the POA, the periventricular area, which consisted of the cells immediately adjacent to the third ventricle, the MPO and the LPO were marked for analysis (Fig. 3B). In the MBH, cellular expression in the arcuate nucleus (ARC) and ventromedial nucleus (VMN) were quantified (Fig. 3C).
Figure 3.
Film autoradiographs from a 35S-labeled probe revealed expression of L-PGDS mRNA in the parenchyma and, strongly, in the leptomeninges in control animals. (A) In the rostral POA, the area around the medial preoptic nucleus (MPO) and the VLPO showed the greatest level of expression. (B) Expression was still detected in the more caudal portions of the MPO and the lateral preoptic area (LPO). (C) In the hypothalamus, expression was most notable in the vl-VMN. (D) There was no specific hybridization signal when the control sense riboprobe was used.
In the POA, E2 treatment reduced L-PGDS expression in the VLPO (Figs. 4A and 5A; F(1,22) = 3.73, P < 0.001) and the periventricular region (Fig. 5B; F(1,30) = 7.68; *, P < 0.01). Moreover, in the VLPO, there was a significant decrease in the mean number of cells per 950 μM2 that expressed the L-PGDS transcript [data not shown; vehicle-treated (23.7 ± 1.8 SEM cells per 950 μM2) vs. E2-treated (16.6 ± 2.0 SEM cells per 950 μM2); F(1,35) = 6.64; P < 0.01, ANOVA]. In contrast, in the MBH, E2 treatment increased L-PGDS expression in the ventrolateral (vl)-VMN and ARC (Figs. 4B and 5C; F(1,19) = 15.97, P < 0.001). These regions also demonstrated an increase in the number of cells per 950 μM2 expressing the transcript [data not shown; ARC: vehicle-treated (4.1 ± 0.3 SEM cells per 950 μM2) vs. E2-treated (7.5 ± 1.5 SEM cells per 950 μM2) vl-VMN: vehicle-treated (6.7 ± 0.9 SEM cells per 950 μM2) vs. E2-treated (11.9 ± 1.2 SEM cells per 950 μM2); F(1,27) = 129.0, P < 0.001, ANOVA]. In addition to quantifying the cellular expression in the parenchyma, the density of reduced silver grains was also quantified within the leptomeninges of the various brain regions. L-PGDS expression was significantly altered only in the leptomeninges of the medial POA region after E2 treatment, with a 2-fold reduction observed compared with vehicle controls (Fig. 5D; F(1,49) = 7.19, P < 0.01).
Figure 4.
Brightfield photomicroscopy of L-PGDS expression revealed the presence of reduced silver grains over counterstained cells in the areas mentioned above, but not when hybridized with the sense probe (data not shown). Differences in the cellular expression of the L-PGDS transcripts between vehicle (Veh) and E2-treated animals were apparent in the VLPO (Upper) and vl-VMN (Lower). Comparing VLPO and the vl-VMN, E2 regulation was in the opposite direction. Representative L-PGDS+ cells considered for quantification are designated with arrows. (Bar = 50 μm.)
Figure 5.
Quantitative analysis of emulsion autoradiography for L-PGD2S expression in the rostral POA (A), the caudal POA (B), the MBH (C), and the leptomeninges (D). In the POA, E2 treatment reduced L-PGD2S expression in the VLPO (*, P < 0.001, ANOVA), the periventricular region (*, P < 0.01, ANOVA), and the leptomeninges of the medial POA region (*, P < 0.01, ANOVA). In contrast, in the MBH, E2 treatment increased L-PGD2S expression in the ARC and vl-VMN (*, P < 0.001, ANOVA).
L-PGDS Immunoreactivity Is Present in VLPO Neurons as Well as Oligodendrocytes.
Immunocytochemical detection of L-PGDS protein was present in the brain regions where the mRNA transcripts were detected (data not shown). Previous studies have reported L-PGDS protein to be localized mainly in the cytoplasm of oligodendrocytes and perivascular cells in the parenchyma of the adult brain (21, 22). L-PGDS has been reported to be in neurons of the developing neonatal rat brain, but its expression switches in adulthood to being localized primarily in oligodendrocytes, except for a few neurons in cortical layers I and II (23). Immunocytochemical analyses of our mouse brain tissue confirmed the expression of L-PGDS primarily in oligodendrocytes, as indicated by their size (<10 μm), pattern of dark cytoplasmic staining, and round compact shape (Fig. 6A). Moreover, this expression was present in, but not limited to, the MBH, POA, and cortex (Fig. 6 A–C). Strikingly, in the VLPO, L-PGDS immunoreactivity was also detected in cells that were large, fusiform, and neuronal in appearance (Fig. 6A). The morphology of these cells indicated that they are neurons and, in fact, they were immunopositive for NeuN, a neuron-specific nuclear marker (Fig. 6D). Interestingly, this hitherto unknown colocalization was not present in other cell groups (Fig. 6 A–C) and may represent a unique mechanism by which PGD2 or L-PGDS, itself, regulates sleep.
Figure 6.
L-PGDS immunoreactivity is present in VLPO neurons as well as oligodendrocytes. Based on the size and shape of the stained structures, immunocytochemistry confirms the expression of L-PGDS in oligodendrocytes (arrowhead) throughout several brain regions. (A) In the VLPO region, oligodendrocytes (arrowheads) as well as cells that were larger, fusiform, and neuronal in appearance (four representative cells shown, with one being noted by the arrow) stained positive for L-PGDS. No specific staining pattern was observed in tissue incubated with the preabsorbed L-PGDS antibodies (data not shown). (B) VLPO, double immunoreactivity for NeuN (arrows; blue-gray) and L-PGDS immunoreactivity (arrowhead; brown) demonstrates that L-PGDS is localized in a subpopulation of neurons (*). This colocalization was not present in other brain regions (see C and D). Cortex (C) and VMN (D), double immunoreactivity for NeuN (arrow) and L-PGDS (arrowhead). There was no colocalization either in cortex or in VMN. Based on morphological profiles, the L-PGDS immunoreactivity present in these areas is restricted to oligodendrocytes that lie in close proximity to the neighboring neurons. (Bar = 50 μm.)
Discussion
L-PGDS catalyzes the production of PGD2, a prostanoid involved in a variety of functions including sedation and sleep. We were interested in a potential regulation by E2 as demonstrated by our microarray analysis of the MBH. Northern blot analysis not only confirmed the induction by E2 in the MBH, but also demonstrated a dramatic suppression by E2 in the POA. Subsequent in situ hybridization confirmed the Northern results and, in addition, revealed a highly specific regulation within the POA such that the reduction of L-PGDS expression in the presence of E2 was limited to the caudal periventricular region and the VLPO cell group. This finding is significant because the VLPO is a putative sleep center in the POA and may suggest an estrogenic component of sleep regulation in adult female mice. Moreover, our finding that L-PGDS is found in a subpopulation of neurons in the VLPO is hitherto unknown and may represent a unique mechanism by which PGD2 or L-PGDS, itself, regulates sleep.
L-PGDS is a member of the protein superfamily of lipocalins (24, 25). Lipocalins are small secretory proteins classified by their ability to bind and transport a myriad of lipophilic molecules that include but are not limited to hormones, bilin, and sexual pheromones (reviewed in ref. 26). Thus, L-PGDS is unique in that it serves a dual function: (i) it is the first enzyme classified in the lipocalin family (24), and (ii) it is a well characterized carrier molecule of retinoids and thyroid hormones (27).
Enzymatically, L-PGDS catalyzes the conversion of PGH2 to PGD2 and is considered to be the sole source of PGD2 in the CNS (28). PGD2 is the major prostanoid produced in the CNS of rodents and humans and functions as a neuromodulator in a variety of CNS actions. These include the modulation of both pain (29, 30) and odor response (31), as well as body temperature regulation (32). However, PGD2 is best known as a potent endogenous somnogen acting at the level of the POA (reviewed in ref. 33).
Seminal work by Nauta (34) led to the POA as a proposed sleep center in the rodent brain. More recent microinjection studies of PGD2 postulated that the site of action for its sleep-promoting effects was located in or near the POA (14, 35). Sherin et al. (36) have identified a discrete cluster of neurons within the VLPO that may play a critical role in the generation of sleep. Indeed, infusion of PGD2 into the subarachnoid space just anterior to the POA (where we detect estrogenic regulation) not only increases nonrapid eye movement sleep (NREM) but also induces striking Fos immunoreactivity (a useful marker of cellular activation) in neurons of the VLPO and basal leptomeninges, suggesting that PGD2 may stimulate sleep-active VLPO neurons (37).
Sleep homeostasis fluctuates over the estrous cycle in female rats and mice such that during the proestrous period when E2 levels are highest, sleep is typically reduced and motor activity is increased (38, 39). Our finding that E2 so profoundly depressed L-PGDS mRNA expression in the VLPO 24 h after treatment (timing analogous to the presorts dark period) is consistent with the idea that preovulatory estrogen surges direct the suppression of the gene in intact cycling animals. Moreover, it is well established that estrogens increase activity levels in rodents (40–44). Female rats have the peak of their activity on proestrus when estrogen levels have been at their highest. In fact, studies have demonstrated that the POA is a key site for the estrogenic regulation of activity and locomotion (45). Recently, Morgan and Pfaff (46, 47) have extended this finding to mice such that E2-treated females demonstrated increased running wheel activity as well as general home cage activity that included rearing, grooming, and burying. These data would indicate that E2 has an arousing effect allowing for increased locomotor behaviors, a component of estrogen-facilitated courtship responses. In light of our discovery that, in the VLPO, E2 suppress the transcript levels of L-PGDS, it is tempting to speculate that a subsequent decrease in the PGD2 may contribute to the general arousal mediated by estrogens.
Based on the evidence that infusion of PGD2 into the parenchyma is less effective at inducing NREM (35), and that PGD2 receptors are located almost exclusively in the leptomeninges, it has been suggested that PGD2 may promote sleep by inducing leptomeningeal cells to release paracrine-signaling molecules such as adenosine, which subsequently excite nearby sleep-active VLPO neurons (reviewed in ref. 48). Interestingly, our finding that L-PGDS protein is localized in a subpopulation of VLPO neurons of adult mice may represent an alternative mechanism by which PGD2/L-PGDS promotes sleep and controls other CNS functions. Because L-PGDS is a secreted protein, it is unclear if the L-PGDS detected in the VLPO neurons was endogenously expressed or endocytosed from the extracellular environment. In either case, the presence of L-PGDS in neurons indicates that PGD2 could behave like a neuroendocrine regulator that is being released to act on neighboring cells. In addition, because L-PGDS is member of the lipocalin family, it may be secreted by the oligodendrocytes, serving as a carrier protein for PGD2 and/or other lipophilic molecules, and presenting them to the neighboring neurons.
In summary, our discovery that E2 regulates the transcript levels of L-PGDS in the rodent brain strongly suggests that PGD2 and/or L-PGDS itself plays a role in E2-regulated actions that include both reproductive and nonreproductive behaviors.
Acknowledgments
We thank Dr. Marlene Schwanzel-Fukuda for providing the paraffin-embedded mouse brains as well as her expertise in tissue fixation and immunocytochemistry.
Abbreviations
- MBH
medial basal hypothalamus
- E2
estradiol
- PG
prostaglandin
- L-PGDS
lipocalin-PG D2 synthase
- POA
preoptic region
- MPO
medial preoptic area
- VLPO
ventrolateral preoptic area
- VMN
ventromedial nucleus of the hypothalamus
- vl-VMN
ventrolateral-VMN
References
- 1.Bauer-Dantoin A C, Knox K L, Schwartz N B, Levine J E. Endocrinology. 1993;133:2413–2417. doi: 10.1210/endo.133.6.8243258. [DOI] [PubMed] [Google Scholar]
- 2.Knobil E, Neill J D. The Physiology of Reproduction. New York: Raven; 1994. [Google Scholar]
- 3.Strobl F J, Levine J E. Endocrinology. 1988;123:622–630. doi: 10.1210/endo-123-1-622. [DOI] [PubMed] [Google Scholar]
- 4.Xu M, Hill J W, Levine J E. Neuroendocrinology. 2000;72:263–271. doi: 10.1159/000054595. [DOI] [PubMed] [Google Scholar]
- 5.Hull E, Meisel R, Sachs B. In: Hormones Brain and Behavior. Pfaff D W, editor. San Diego: Academic; 2002. [Google Scholar]
- 6.Pfaff D W. Drive: Neurobiological and Molecular Mechanisms of Sexual Motivation. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 7.Fillit H, Luine V. Maturitas. 1997;26:159–164. doi: 10.1016/s0378-5122(97)01101-8. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs R B. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 9.Luine V N, Richards S T, Wu V Y, Beck K D. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 10.Dubal D B, Shughrue P J, Wilson M E, Merchenthaler I, Wise P M. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linford N, Wade C, Dorsa D. J Neurocytol. 2000;29:367–374. doi: 10.1023/a:1007113323582. [DOI] [PubMed] [Google Scholar]
- 12.Singer C A, Rogers K L, Strickland T M, Dorsa D M. Neurosci Lett. 1996;212:13–16. doi: 10.1016/0304-3940(96)12760-9. [DOI] [PubMed] [Google Scholar]
- 13.Mong J A, Krebs C, Pfaff D W. Endocrinology. 2002;143:2002–2006. doi: 10.1210/endo.143.6.8866. [DOI] [PubMed] [Google Scholar]
- 14.Ueno R, Ishikawa Y, Nakayama T, Hayaishi O. Biochem Biophys Res Commun. 1982;109:576–582. doi: 10.1016/0006-291x(82)91760-0. [DOI] [PubMed] [Google Scholar]
- 15.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: Academic; 1997. [Google Scholar]
- 16.Lockhart D J, Dong H, Byrne M C, Follettie M T, Gallo M V, Chee M S, Mittmann M, Wang C, Kobayashi M, Horton H, Brown E L. Nat Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 18.Simeone A. In: In Situ Hybridization: A Practical Approach. Wilkinson D G, editor. New York: Oxford Univ. Press; 1998. pp. 69–86. [Google Scholar]
- 19.Schwanzel-Fukuda M, Abraham S, Crossin K L, Edelman G M, Pfaff D W. J Comp Neurol. 1992;321:1–18. doi: 10.1002/cne.903210102. [DOI] [PubMed] [Google Scholar]
- 20.Mong J A, McCarthy M M, Nunez J L. J Neuroendocrinol. 2002;14:45–55. doi: 10.1046/j.1365-2826.2002.00737.x. [DOI] [PubMed] [Google Scholar]
- 21.Beuckmann C T, Lazarus M, Gerashchenko D, Mizoguchi A, Nomura S, Mohri I, Uesugi A, Kaneko T, Mizuno N, Hayaishi O, Urade Y. J Comp Neurol. 2000;428:62–78. doi: 10.1002/1096-9861(20001204)428:1<62::aid-cne6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Urade Y, Kitahama K, Ohishi H, Kaneko T, Mizuno N, Hayaishi O. Proc Natl Acad Sci USA. 1993;90:9070–9074. doi: 10.1073/pnas.90.19.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urade Y, Fujimoto N, Kaneko T, Konishi A, Mizuno N, Hayaishi O. J Biol Chem. 1987;262:15132–15136. [PubMed] [Google Scholar]
- 24.Nagata A, Suzuki Y, Igarashi M, Eguchi N, Toh H, Urade Y, Hayaishi O. Proc Natl Acad Sci USA. 1991;88:4020–4024. doi: 10.1073/pnas.88.9.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toh H, Yokoyama C, Tanabe T, Yoshimoto T, Yamamoto S. Prostaglandins. 1992;44:291–315. doi: 10.1016/0090-6980(92)90004-d. [DOI] [PubMed] [Google Scholar]
- 26.Flower D R. Biochem J. 1996;318:1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beuckmann C T, Aoyagi M, Okazaki I, Hiroike T, Toh H, Hayaishi O, Urade Y. Biochemistry. 1999;38:8006–8013. doi: 10.1021/bi990261p. [DOI] [PubMed] [Google Scholar]
- 28.Urade Y, Watanabe K, Hayaishi O. J Lipid Mediat Cell Signalling. 1995;12:257–273. doi: 10.1016/0929-7855(95)00032-l. [DOI] [PubMed] [Google Scholar]
- 29.Eguchi N, Minami T, Shirafuji N, Kanaoka Y, Tanaka T, Nagata A, Yoshida N, Urade Y, Ito S, Hayaishi O. Proc Natl Acad Sci USA. 1999;96:726–730. doi: 10.1073/pnas.96.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horiguchi S, Ueno R, Hyodo M, Hayaishi O. Eur J Pharmacol. 1986;122:173–179. doi: 10.1016/0014-2999(86)90100-7. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe Y, Mori K, Imamura K, Takagi S F, Hayaishi O. Brain Res. 1986;378:216–222. doi: 10.1016/0006-8993(86)90924-8. [DOI] [PubMed] [Google Scholar]
- 32.Ueno R, Narumiya S, Ogorochi T, Nakayama T, Ishikawa Y, Hayaishi O. Proc Natl Acad Sci USA. 1982;79:6093–6097. doi: 10.1073/pnas.79.19.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urade Y, Hayaishi O. Biochim Biophys Acta. 1999;1436:606–615. doi: 10.1016/s0005-2760(98)00163-5. [DOI] [PubMed] [Google Scholar]
- 34.Nauta W J H. J Neurophysiol. 1946;9:285–316. doi: 10.1152/jn.1946.9.4.285. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura H, Nakajima T, Osaka T, Satoh S, Kawase K, Kubo E, Kantha S S, Kasahara K, Hayaishi O. Proc Natl Acad Sci USA. 1994;91:11998–12002. doi: 10.1073/pnas.91.25.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherin J E, Shiromani P J, McCarley R W, Saper C B. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 37.Scammell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Proc Natl Acad Sci USA. 1998;95:7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwierin B, Borbely A A, Tobler I. Brain Res. 1998;811:96–104. doi: 10.1016/s0006-8993(98)00991-3. [DOI] [PubMed] [Google Scholar]
- 39.Fang J, Fishbein W. Brain Res. 1996;734:275–285. [PubMed] [Google Scholar]
- 40.Wade G N, Zucker I. J Comp Physiol Psychol. 1970;72:328–336. doi: 10.1037/h0029461. [DOI] [PubMed] [Google Scholar]
- 41.Gerall A A, Napoli A M, Cooper U C. Physiol Behav. 1973;10:225–229. doi: 10.1016/0031-9384(73)90302-8. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy G. J Physiol. 1964;172:383–392. doi: 10.1113/jphysiol.1964.sp007426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas D K, Storlien L H, Bellingham W P, Gillette K. Physiol Behav. 1986;36:567–573. doi: 10.1016/0031-9384(86)90332-x. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz de Elvira M C, Persaud R, Coen C W. Physiol Behav. 1992;52:277–284. doi: 10.1016/0031-9384(92)90271-3. [DOI] [PubMed] [Google Scholar]
- 45.Fahrbach S E, Meisel R L, Pfaff D W. Physiol Behav. 1985;35:985–992. doi: 10.1016/0031-9384(85)90270-7. [DOI] [PubMed] [Google Scholar]
- 46.Morgan M A, Pfaff D W. Behav Brain Res. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 47.Morgan M A, Pfaff D W. Horm Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- 48.Hayaishi O. J Appl Physiol. 2002;92:863–868. doi: 10.1152/japplphysiol.00766.2001. [DOI] [PubMed] [Google Scholar]