Abstract
It has been demonstrated previously that both the cystic fibrosis transmembrane conductance regulator (CFTR) and β2 adrenergic receptor (β2AR) can bind ezrin/radixin/moesin-binding phosphoprotein 50 (EBP50, also referred to as NHERF) through their PDZ motifs. Here, we show that β2 is the major adrenergic receptor isoform expressed in airway epithelia and that it colocalizes with CFTR at the apical membrane. β2AR stimulation increases CFTR activity, in airway epithelial cells, that is glybenclamide sensitive. Deletion of the PDZ motif from CFTR uncouples the channel from the receptor both physically and functionally. This uncoupling is specific to the β2AR receptor and does not affect CFTR coupling to other receptors (e.g., adenosine receptor pathway). Biochemical studies demonstrate the existence of a macromolecular complex involving CFTR-EBP50-β2AR through PDZ-based interactions. Assembly of the complex is regulated by PKA-dependent phosphorylation. Deleting the regulatory domain of CFTR abolishes PKA regulation of complex assembly. This report summarizes a macromolecular signaling complex involving CFTR, the implications of which may be relevant to CFTR-dysfunction diseases.
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-regulated Cl− channel found on the apical surface of many epithelia, including the airways, sweat gland, and gastrointestinal tract (1). Gating of the channel is tightly regulated through phosphorylation of the regulatory or R domain, which is accomplished primarily by elevation of cAMP and activation of PKA (2). The CFTR Cl− channel may contribute to either absorptive or secretory ion and fluid transport, which is also dependent on the organ system and driving forces prevailing in the tissue environment (3). For example, CFTR is an important component of net fluid absorption along the airway surface, but is believed to primarily regulate anion and fluid secretion in the underlying submucosal glands. Defects in CFTR disrupt both of these processes, and are believed to be primarily responsible for the ion transport defects and clinical findings in cystic fibrosis (4).
Regulation of the CFTR protein in vivo is believed to be accomplished through activation of surface receptors that couple to adenyl cyclase and raise cellular cAMP (5). This result has been clearly demonstrated for the β adrenergic receptor, as stimulation of the human airway mucosa with the nonspecific β receptor agonist isoproterenol activates CFTR-dependent Cl− transport in vivo (6). Biochemical studies have demonstrated that the C-termini of CFTR and β2 adrenergic receptors (β2AR) possess PDZ binding motifs, and these domains contribute to interactions between these membrane proteins (7–9) and cytoskeletal proteins (10) that are important for channel regulation (11, 12) and trafficking (13, 14). No studies, however, have conclusively demonstrated that interactions of this sort contribute to CFTR regulation by surface receptors.
In this report, we investigated biochemical and functional interactions involving CFTR and the β2AR. Our findings indicate that PDZ-based interactions with ezrin/radixin/moesin-binding phosphoprotein 50 (EBP50) govern both physical and functional regulation of CFTR by β2 AR. Subsequent studies identify the necessary components of this macromolecular signaling complex, and reveal PKA-dependent regulation of complex assembly. These studies clarify the nature of CFTR regulation by β2AR, and demonstrate an additional level of extrinsic protein regulation through binding partner interactions and phosphorylation status.
Materials and Methods
Materials and Constructs.
Bacterial expression vectors were obtained from Amersham Pharmacia (pGEX) and New England Biolabs (pMAL). Eukaryotic expression vectors were purchased from Invitrogen (pCDNA-3). CFTR C-terminal antibody GA-1 (epitope 1430–1460) has been described (15). Monoclonal EBP50 antibody was obtained from Transduction Laboratories, and epitope affinity-purified polyclonal antibody against EBP50 (his-tagged fusion protein) was from Affinity BioReagents (Golden, CO). Polyclonal β2AR antibodies were from Santa Cruz Biotechnology. Calu-3 and Cos-7 cells were obtained from American Type Culture Collection. Baby hamster kidney (BHK) cells stably transfected with CFTR and CFTRhis10 have been described (16)
Colocalization of CFTR and β2AR.
Fully polarized monolayers of calu-3 cells were biotinylated apically or basolaterally by using 0.5 mg/ml sulfo-NHS-LC-biotin (Pierce) at 4°C for 30 min. Monolayers from six permeable supports were scraped off and homogenized, and the plasma membrane was separated on a sucrose cushion by ultracentrifugation as described (17). Streptavidin agarose beads (20 μl) were used to capture the biotinylated membranes and subjected to Western blot analysis. Syntaxin 3 was used as an apical marker (18) and Na+-K+-Cl− Cotransporter (NKCC) was used as a basolateral marker (19).
Macromolecular Complex Assembly.
This assay was performed by using maltose-binding protein (MBP)-β2AR-C tail fusion protein (0–1 μM) immobilized on amylose beads (20 μl) and incubated with GST-EBP50. The binding was done in 200 μl of lysis buffer (PBS–0.2% Triton X-100 + protease inhibitors) and mixed at 22°C for 2 h. The complex was washed once with the same buffer and allowed to bind CFTR or CFTRhis10 from lysates of BHK cells. The binding was done at 4°C for 3 h with constant mixing. The protein complex was washed twice and probed for CFTR by immunoblotting (GA1 monoclonal, ref. 15).
Transient Transfection.
Cells were transfected by using Lipofectamine 2000 (Invitrogen). Briefly, 1 μg of DNA was incubated in 600 μl of DMEM (without FBS or antibiotics); then, 6 μl of Lipofectamine 2000 was added and incubated for 40 min. BHK cells (≈80% confluent; 60-mm dishes) were washed once with DMEM and incubated with 1.5 ml of DMEM. The DNA-lipid mix was added to the plates and incubated for 6 h, at the end of which 5 ml of FBS containing media was added. The next day, fresh medium was added, and the cells were incubated for 48 h before use.
Other Methods.
Immunofluorescent localization of β2AR at the apical surface of polarized monolayers of calu-3 cells was performed according to Prince et al. (20). Iodide efflux was performed as described before (21). Halide transport using a fluorescent indicator dye (SPQ) was performed as described (22). Anion secretion of polarized epithelial monolayers mounted in an Ussing chamber was performed according to Smith et al. (23).
Results and Discussion
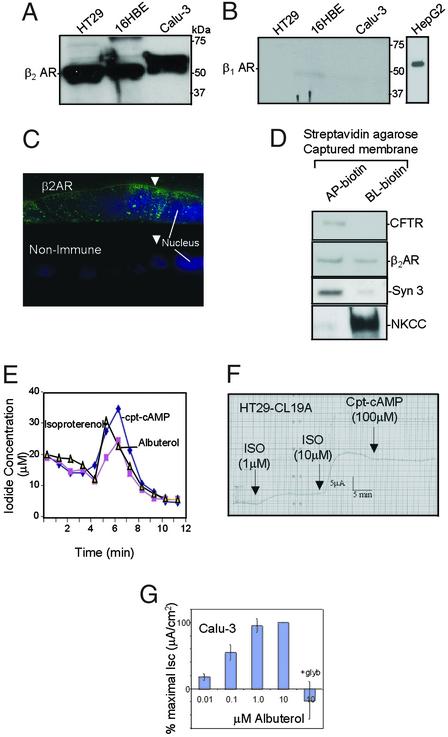
β2AR appeared as a major immunoreactive band of ≈55 kDa in epithelial cells lining the airway and gut (Fig. 1A). By contrast, β1AR was not detectable in these cells (Fig. 1B; HepG2 cells were used as positive control). In polarized calu-3 epithelial cell monolayers, β2AR was significantly expressed at or near the apical cell surface (Fig. 1C; arrows indicate apical surfaces). Staining was also observed at the lateral and basal surfaces and at numerous intracellular puncta. There was no staining with nonimmune control IgG (Fig. 1C Lower). Apical colocalization of CFTR with β2AR was also demonstrated biochemically in polarized calu-3 cells (see Materials and Methods for details). Apically biotinylated membranes were captured by using streptavidin agarose containing both CFTR and β2AR (Fig. 1D). Basolateral membranes also contained β2AR without detectable CFTR (Fig. 1D). Syntaxin 3 was used as an apical marker, and Na+-K+-Cl− Cotransporter as a basolateral marker (Fig. 1D; refs. 18 and 19). Iodide efflux was stimulated on treating nonpolarized calu-3 cells with albuterol (β2AR selective agonist; ref. 24); isoproterenol (mixed β1AR and β2AR agonist; ref. 25) and cpt-cAMP mixture is shown in Fig. 1E. A dose-dependent increase in short-circuit currents was observed on addition of isoproterenol to the apical side of polarized HT29-CL19A cells (Fig. 1F). These currents did not increase further after adding 100 μM cpt-cAMP. Addition of albuterol to the apical surface of polarized calu-3 cell monolayers caused a concentration-dependent increase in short-circuit current that was glybenclamide sensitive (CFTR blocker; Fig. 1G). Cumulatively, these results provide clear evidence that β2AR are present at the apical surface of airway and colonic epithelial cells, and that β2AR-selective agonists activate CFTR-dependent Cl− currents at the apical surface of these epithelial cells.
Figure 1.
β2AR is expressed at the apical surface of airway epithelial cells and can physically and functionally interact with CFTR. (A) Western blot analysis with affinity-purified β2AR antibodies (Santa Cruz Biotechnology; 1 μg/ml) shows that β2AR is expressed in HT29-CL19A (colonic epithelial), 16HBE14o− (human bronchial epithelial), and calu-3 (serous gland epithelial) cell lysates (100 μg per lane). (B) β1AR is not detected in these cells using affinity-purified β1AR antibody (Santa Cruz Biotechnology; 1 μg/ml; HepG2 cell lysate was used as positive control). (C) Immunofluorescence localization of β2AR at the apical membrane in calu-3 cells grown on a permeable supports. Polarized monolayers were fixed and stained after 8 days in culture as described (20). AP, apical; BL, basolateral. Arrows indicate the apical membrane. The nucleus was stained with Hoechst reagent (20). (D) Colocalization of CFTR and β2AR in apical membranes of polarized calu-3 cells (see Materials and Methods). (E) Iodide effluxes were measured by using calu-3 monolayers (n = 2) according to described methods (21). Cells were activated after 4 min with cpt-cAMP mixture [200 μM cpt-cAMP/10 μM forskolin/1 mM 3-isobutyl-1-methylxanthine (IBMX)], isoproterenol (50 μM), or albuterol (100 μM). (F) Activation of dose-dependent anion secretion by adding isoproterenol to the apical surfaces of polarized HT29-CL19A cells mounted in an Ussing chamber (23). The currents could not be potentiated further by cpt-cAMP. (G) Activation of glibenclamide-sensitive anion secretion by β2AR agonist added to the apical side of calu-3 monolayers mounted in Ussing chambers (area = 0.33 cm2; n = 4; ref. 23). Cells were treated with apical albuterol (0–10 μM), followed by apical glybenclamide (200 μM).
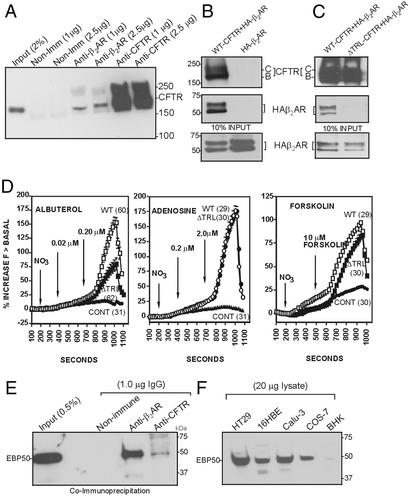
β2AR-specific antibodies coimmunoprecipitated CFTR from calu-3 cells (Fig. 2A). Similarly, in an overexpression system, we observed that CFTR antibodies coimmunoprecipitated hemagglutinin-tagged β2AR (HA-β2AR; ref. 26) when coexpressed with CFTR in Cos-7 cells but not in the absence of CFTR (Fig. 2B). Deletion of the PDZ binding motif of CFTR (last 3 aa and referred to as ΔTRL-CFTR) eliminated the physical interaction between the channel and receptor (Fig. 2C). The input is shown in Fig. 2 B and C Middle and Bottom. These results suggest that the PDZ motif of CFTR is essential for the physical interaction between CFTR and the β2AR. Removal of the PDZ motif (ΔTRL-CFTR) specifically reduced efflux after β2AR stimulation after transient expression in Cos-7 cells (Fig. 2D Left). In contrast, activation of CFTR by agonists such as adenosine (27) or forskolin (28), both of which elevate cAMP without by using the β2AR signaling pathway, were not affected by deletion of the CFTR PDZ motif (Fig. 2D Center and Right). These observations make two important points. First, the PDZ motif is essential for physical and functional coupling of CFTR Cl− channels to the β2AR. Second, CFTR can be activated by multiple signaling pathways.
Figure 2.
Physical and functional interaction between CFTR and β2AR is mediated by the PDZ motif of CFTR. (A) CFTR coimmunoprecipitates with β2AR from calu-3 cells. Ten confluent 100-mm dishes were lysed in PBS–0.2% Triton X-100, and CFTR was immunoprecipitated by using a polyclonal CFTR antibody (raised against amino acid residues 521–828) cross-linked to protein A/G beads (11). (B) Coimmunoprecipitation of CFTR and β2AR in Cos-7 cells expressing recombinant CFTR and β2AR (vaccinia expression system; ref. 35). The blot was cut at an appropriate position and probed by using the monoclonal antibodies GA-1 (recognizes amino acids 1430–1460 of CFTR; Top) and anti-HA tag mAb (Sigma; Middle). (C) Deletion of the PDZ motif (ΔTRL-CFTR) abolished the interaction of CFTR with HA-β2AR. The vaccinia expression system generated approximately equal steady-state levels of mature (band C) and immature (band B) CFTR. (D) Halide transport by ΔTRL-CFTR expressed in Cos-7 cells is reduced on stimulation by β2AR agonist (albuterol, Left), compared with WT-CFTR (Left). In contrast, deletion of the TRL motif of CFTR had no effect on halide transport stimulated by adenosine receptor agonist, shown in Center, and forskolin, shown at Right, compared with WT-CFTR. There was no significant difference in the average basal level of halide transport in the absence of stimulation of WT-CFTR vs. ΔTRL-CFTR. The total protein levels were not altered (data not shown). Fluorescence of the halide-sensitive indicator dye (SPQ) was assayed as described (22). (E) Cross-linked β2AR IgG (1 μg), polyclonal CFTR IgG (1 μg), or nonimmune IgG (1 μg, Santa Cruz Biotechnology) was used to immunoprecipitate 16HBE14o− cell lysate (PBS–0.2% Triton X-100). The blots were probed by using monoclonal EBP50 antibody (Transduction Laboratories; 2 μg/ml). (F) Western blot analysis of lysates of various cell lines using affinity-purified EBP50 polyclonal antibody (see Materials and Methods). The protein was detected (EBP50) in all cell lines tested.
Given that the physical interaction between CFTR and the β2AR requires the C-terminal PDZ motif TRL of CFTR, we investigated the possible involvement of EBP50 in molecular coupling. This protein has previously been shown to bind to the PDZ motifs of both CFTR and β2AR (7, 8). β2AR and CFTR-specific antibodies were able to coimmunoprecipitate EBP50 from human bronchial epithelial cells (16HBE14o−), whereas nonimmune rabbit IgG was negative (Fig. 2E). To see whether each of the cell lines used in this study expressed EBP50, an affinity-purified polyclonal antibody raised against the entire fusion protein was used (see Materials and Methods). Results shown in Fig. 2F reveal that all of the cell lines used in this study expressed EBP50. Assuming that the antibody recognizes hamster and primate EBP50 equally well, the data in Fig. 2F indicate that BHK cells had a much lower level of expression of EBP50 compared with HT29, 16HBE, calu-3, and Cos-7 cells.
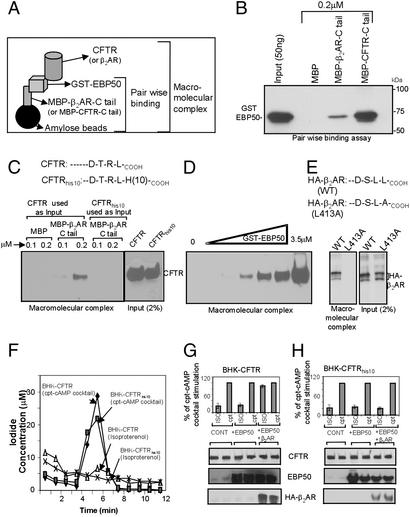
To investigate the nature of these interactions in detail, we developed an in vitro assay to assemble the macromolecular complex (Fig. 3A). The first step was to form a complex between the C-terminal tail of CFTR or C-terminal tail of β2AR and EBP50 (this step is also referred to as pair-wise binding; Fig. 3A). As reported earlier (7, 8), EBP50 binding to CFTR and β2AR was direct and is demonstrated by this pair-wise binding assay (Fig. 3B). A macromolecular complex of β2AR, EBP50, and CFTR assembled spontaneously when the components were combined in vitro. First, a complex between MBP-β2AR-C tail (0.5 μM) and GST-EBP50 (0.5 and 1.0 μM) was formed. After washing, this complex was mixed with the lysate of BHK cells expressing either CFTR or CFTRhis10 (the His tag is present on the C terminus, and disrupts the binding of PDZ domains; data not shown). With this approach, our results demonstrated the existence of a macromolecular complex between MBP-β2AR-C tail, GST-EBP50, and CFTR (Fig. 3C). MBP alone failed to complex with CFTR. Additionally, CFTRhis10 failed to form a complex (Fig. 3C). Dose-dependent macromolecular complex formation was observed through increases in the EBP50 concentration (Fig. 3D). All of the in vitro binding studies were performed under nonsaturating conditions. A macromolecular complex was formed by using MBP-CFTR-C tail, GST-EBP50, and HA-β2AR (Fig. 3E). The mutant β2AR that could not bind to the PDZ domain (HA-β2AR-L413A; ref. 29) also failed to form a complex (Fig. 3E).
Figure 3.
Macromolecular complex of CFTR and β2AR is mediated by EBP50. (A) Pictorial representation of the in vitro macromolecular assembly (see Materials and Methods). (B) C-terminal tail of CFTR and β2AR interacted directly with EBP50 as determined by pair-wise binding assay. MBP-fusion proteins (0.02 μM) were used to bind 0.001 μM GST-EBP50 for 60 min at 22°C in lysis buffer. The complex was pulled down by amylose beads (20 μl) and washed twice with the same buffer. The complex was blotted and probed by using anti-EBP50 monoclonal antibody. (C) Macromolecular complex of β2AR, EBP50, and CFTR. A complex between MBP-β2AR-C tail (0.5 μM) and GST-EBP50 (0.5 and 1.0 μM) was formed in lysis buffer (200 μl final volume) as described above, washed with 1 ml of the same buffer, and mixed with 1 ml of BHK cell lysate (PBS–0.2% Triton X-100) containing CFTR or CFTRhis10. The proteins were bound for 3 h at 4°C, washed twice with the same buffer, and subjected to blotting by using GA1 mAb (11). BHK cells stably expressing CFTR contained mainly the complex glycosylated mature “band C” form. The inputs are shown at Right. (D) Dose-dependent complex formation with increasing amounts of EBP50. Saturation was not observed up to 3.5 μM. (E) Formation of a macromolecular complex MBP-CFTR-C tail (0.5 μM), GST-EBP50 (1.0 μM), and HA-β2AR (WT and mutant; L413A). (F) BHK cells expressing CFTR or CFTRhis10 did not respond to isoproterenol (100 μM) or albuterol (data not shown). These cells responded to cpt-cAMP mixture. (G) BHK-CFTR cells were transiently transfected with 1 μg of EBP50 with or without HA-β2AR cDNA (Lipofectamine 2000; see Materials and Methods) and subjected to iodide efflux by using isoproterenol (ISO) or the cpt-cAMP (cpt) mixture. The data are presented as the percentage of cpt-cAMP mixture stimulation (at 5-min time points). (H) BHK-CFTRhis10 cells were transiently transfected with 1 μg of EBP50 with or without HA-β2AR cDNA and subjected to iodide efflux as described above.
BHK cells expressing CFTR or CFTRhis10 failed to activate halide efflux in response to isoproterenol (Fig. 3F) or albuterol (data not shown). In contrast, iodide efflux was observed in response to a cpt-cAMP mixture (Fig. 3F). In subsequent studies, both of these cell lines (BHK-CFTR and BHK-CFTRhis10) were transiently transfected with EBP50 and HA-β2AR. As shown in Fig. 3G, BHK-CFTR cells expressing EBP50 and HA-β2AR responded to the β receptor agonist (isoproterenol). Similar response was also observed in the presence of HA-β2AR alone (data not shown). In contrast, BHK-CFTRhis10 cells expressing EBP50 and HA-β2AR failed to respond to isoproterenol (Fig. 3H). Together, these results further demonstrate that the receptor (β2AR) and channel (CFTR) are physically and functionally coupled together into a macromolecular signaling complex through interactions with EBP50.
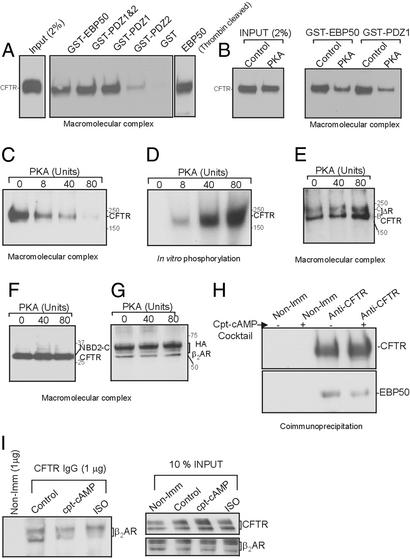
Next, we identified the minimum EBP50 domain required for complex formation. Truncation constructs of EBP50 corresponding to various domains were generated as GST-fusion proteins by PCR. The domains included the full-length protein (GST-EBP50; amino acids 1–358), the first PDZ domain (GST-PDZ1; amino acids 1–97), the second PDZ domain (GST-PDZ2; amino acids 133–244), and finally both PDZ domains (GST-PDZ1 and -2; amino acids 1–244). The details of these clones will be described elsewhere (D.N. and K.L.K., unpublished observation). We assembled macromolecular complexes by using these truncated forms at 1 μM, as described above. Fig. 4A shows the result of one such experiment. GST-EBP50, GST-PDZ1 and -2, and GST-PDZ1 formed a macromolecular complex. GST-PDZ2 did not seem to form a complex (often a weak signal was observed), whereas GST alone was used as negative control (Fig. 4A). It is interesting to note that the first PDZ domain alone was sufficient to link the receptor and CFTR. Previously, Fouassier et al. (30) demonstrated that PDZ domains in isolation can self-associate into multimeric forms, providing multiple PDZ motif binding sites. This action may explain how the first PDZ domain (PDZ1) by itself can associate with both CFTR and β2AR (Fig. 4A). To rule out the possibility of GST having a role in multimerization, PDZ1 was cleaved from GST by using thrombin and used in the macromolecular complex assay. The results shown in Fig. 4A Right demonstrate that binding is retained in the absence of GST. PDZ1 lacking GST can form a complex as well (data not shown).
Figure 4.
Association of CFTR with β2AR and EBP50 complex is regulated by PKA. (A) Macromolecular complex formation between MBP-β2AR-C tail (1 μM) and CFTR by using various EBP50 constructs (1 μM). The assay is described in Materials and Methods. (B) Effect of PKA on macromolecular complex formation. ATP (2 mM), Mg (2 mM), and PKA (80 units) were added to the cell lysate, and the complex was formed by stepwise assembly as described (see Materials and Methods). (C) A dose-dependent inhibitory effect of PKA (0–80 units) on macromolecular complex formation is shown. (D) In vitro phosphorylation of CFTR was performed in parallel, verifying that the protein was being appropriately phosphorylated. (E) Deleting most of the R domain (ΔR-CFTR; ref. 32) eliminated the inhibitory effects of PKA on the complex formation. The vaccinia expression system was used to express ΔR-CFTR, which yielded equal amounts of immature and mature protein (bands B and C). (F) NBD-2-C tail of CFTR (amino acids 1210–1480) also eliminated the PKA inhibitory effects on the macromolecular complex formation (vaccinia expression system was used to generate the protein). (G) PKA phosphorylation did not affect β2AR in the complex of MBP-CFTR-C tail (1 μM) and GST-EBP50 (1 μM). In vitro assembly of the complex is described (see Materials and Methods). Vaccinia expression system was used to generate HA-β2AR. (H) Coimmunoprecipitation of CFTR and EBP50 from calu-3 cells treated with or without cpt-cAMP mixture for 10 min at 37°C. cpt-cAMP treatment diminishes EBP50 binding during coimmunoprecipitation. (I) Coimmunoprecipitation of CFTR and β2AR from COS-7 cells (expressing recombinant CFTR and HA-β2AR) treated with agonists for 10 min at 37°C.
Phosphorylation of the CFTR regulatory (R) domain is essential for channel activation (2). We therefore investigated whether phosphorylation of CFTR had an effect on formation of the macromolecular complex. As shown in Fig. 4B, PKA-dependent phosphorylation strongly inhibited (54%) formation of the macromolecular complex (inputs are shown in Fig. 4B Left). Complexes formed by the first PDZ domain (GST-PDZ1) had the same PKA sensitivity (49% inhibition) as those formed with full-length EBP50 (Fig. 4B). PKA inhibited the ability of CFTR to bind to the complex in a dose-dependent manner (Fig. 4C), and binding was inversely proportional to the phosphorylation of CFTR monitored simultaneously (Fig. 4D). Previous work has demonstrated that most PKA-dependent phosphorylation of CFTR occurs on its R domain (31). Deleting most of the R domain of CFTR (S660A ΔRCFTR; lacking amino acids 708–835; ref. 32) abolished the PKA sensitivity of CFTR binding in the complex (Fig. 4 E and F). This complex was not formed in the absence of GST-EBP50 (negative control; data not shown). Because PKA did not affect the ability of HA-β2AR to form a complex with MBP-CFTR-C tail and EBP50 (Fig. 4G), our results suggest that phosphorylation of the R domain makes the C tail of CFTR unavailable for binding to EBP50. To confirm these in vitro results, we coimmunoprecipitated CFTR and EBP50 in the presence or absence of the cpt-cAMP mixture treatment from calu-3 cells (using anti-CFTR antibodies; Fig. 4H). The amount of EBP50 coimmunoprecipitated decreased by 47% with the treatment of the mixture. Similarly, the agonist (cAMP mixture and Isoproterenol) inhibited the binding of CFTR to β2AR by ≈50% (Fig. 4I). However, the cpt-cAMP mixture (and β2AR agonist) did not significantly alter the binding of EBP50 to the receptor (data not shown).
Our data suggest that sequestration of CFTR and β2AR at the apical surface of epithelial cells is mediated by EBP50. A model based on our observations and the literature is presented in Fig. 5. The present results show that protein–protein interactions (macromolecular complex assembly) are essential for full activation of the channel by the β2AR pathway, which can be circumvented by using other pathways (e.g., adenosine and forskolin; Fig. 2D). These interactions may be critical for a rapid and specific signal transduction from the receptor to the channel in a compartmentalized fashion (Fig. 5; refs. 33 and 34). This report demonstrates cross-talk between receptor and the channel through PDZ-mediated physical interaction. We speculate that interactions of this sort may also be important to CFTR regulation of other ion channels (e.g., ENaC) and other proteins. Our results also show how certain defective forms of CFTR may lead to abnormal CFTR function in the context of receptor-based signaling and signal compartmentalization.
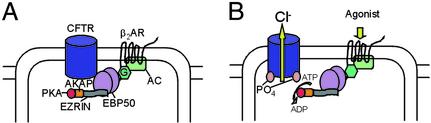
Figure 5.
A model of CFTR-EBP50-β2AR signaling. CFTR, β2AR, and EBP50 can exist as a complex at the apical surface of epithelial cells. G protein (G) can be associated with β2AR (data not shown; ref. 33) and protein kinase A (PKA) anchored to AKAP via ezrin (10) and is likely to be in the complex. On agonist activation of the receptor, adenylate cyclase is stimulated through the Gs pathway (33), leading to an increase in highly compartmentalized cAMP. This increased local concentration of cAMP leads to the activation of PKA, which is in close proximity to CFTR (36), leading to a compartmentalized and specific signaling of the channel. Phosphorylation disrupts the complex, leading to the receptor-based activation of CFTR.
Acknowledgments
We thank Dr. David L. Armbruster (Scientific Publications, University of Tennessee Health Science Center), Dr. Christopher R. Marino (Veterans Affairs Medical Center), and Dr. Partha Krishnamurthy (St. Jude Children's Research Hospital) for critically reading the manuscript. We are grateful to Dr. Marc G. Caron (Duke University) for providing us with HA-β2AR cDNA and William Robinson (University of Tennessee) for technical support. This work was supported by National Institutes of Health Grant DK58545 (to A.P.N.), an American Heart Association Grant in Aid, SE affiliate 0265008B (to A.P.N.), and Cystic Fibrosis Foundation Research Grant NAREN99GO. J.P.C. is a recipient of the Leory Mathews Award from the Cystic Fibrosis Foundation.
Abbreviations
- CFTR
cystic fibrosis transmembrane conductance regulator
- β2AR
β2 adrenergic receptor
- MBP
maltose-binding protein
- HA
hemagglutinin
- R
regulatory
- EBP50
ezrin/radixin/moesin-binding phosphoprotein 50
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Welsh M J, Tsui L-C, Boat T F, Beaudet A L. In: The Metabolic and Molecular Basis of Inherited Diseases: Membrane Transport Systems. Scriver C, Beaudet A L, Sly W S, Valle D, editors. Vol. 3. New York: McGraw–Hill; 1995. pp. 3799–3876. [Google Scholar]
- 2.Gadsby D C, Nairn A C. Adv Second Messenger Phosphoprotein Res. 1999;33:79–106. doi: 10.1016/s1040-7952(99)80006-8. [DOI] [PubMed] [Google Scholar]
- 3.Kunzelmann K. News Physiol Sci. 2001;16:167–170. doi: 10.1152/physiologyonline.2001.16.4.167. [DOI] [PubMed] [Google Scholar]
- 4.Matsui H, Davis C W, Tarran R, Boucher R C. J Clin Invest. 2000;105:1419–1427. doi: 10.1172/JCI4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan H C, Fong S K, So S C, Chung Y W, Wong P Y. J Physiol. 1997;501:517–525. doi: 10.1111/j.1469-7793.1997.517bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker L C, Venglarik C J, Aubin G, Weatherly M R, McCarty N A, Lesnick B, Ruiz F, Clancy J P, Sorscher E J. Am J Respir Crit Care Med. 1997;155:1684–1689. doi: 10.1164/ajrccm.155.5.9154877. [DOI] [PubMed] [Google Scholar]
- 7.Hall R A, Ostedgaard L S, Premont R T, Blitzer J T, Rahman N, Welsh M J, Lefkowitz R J. Proc Natl Acad Sci USA. 1998;95:8496–8501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Short D B, Trotter K W, Reczek D, Kreda S M, Bretscher A, Boucher R C, Stutts M J, Milgram S L. J Biol Chem. 1998;273:19797–197801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Raab R W, Schatz P J, Guggino W B, Li M. FEBS Lett. 1998;427:103–108. doi: 10.1016/s0014-5793(98)00402-5. [DOI] [PubMed] [Google Scholar]
- 10.Brdickova N, Brdicka T, Andera L, Spicka J, Angelisova P, Milgram S L, Horejsi V. FEBS Lett. 2001;507:133–136. doi: 10.1016/s0014-5793(01)02955-6. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Yue H, Derin R B, Guggino W B, Li M. Cell. 200;103:169–179. doi: 10.1016/s0092-8674(00)00096-9. [DOI] [PubMed] [Google Scholar]
- 12.Raghuram V, Mak D D, Foskett J K. Proc Natl Acad Sci USA. 2001;98:1300–1305. doi: 10.1073/pnas.031538898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moyer B D, Duhaime M, Shaw C, Denton J, Reynolds D, Karlson K H, Pfeiffer J, Wang S, Mickle J E, Milewski M, et al. J Biol Chem. 2000;275:27069–27074. doi: 10.1074/jbc.M004951200. [DOI] [PubMed] [Google Scholar]
- 14.Cheng J, Moyer B D, Milewski M, Loffing J, Ikeda M, Mickle J E, Cutting G R, Li M, Stanton B A, Guggino W B. J Biol Chem. 2002;277:3520–3529. doi: 10.1074/jbc.M110177200. [DOI] [PubMed] [Google Scholar]
- 15.Naren A P. Methods Mol Med. 2002;70:175–186. doi: 10.1385/1-59259-187-6:175. [DOI] [PubMed] [Google Scholar]
- 16.Zhu T, Dahan D, Evagelidis A, Zheng S, Luo J, Hanrahan J W. J Biol Chem. 1999;274:29102–29107. doi: 10.1074/jbc.274.41.29102. [DOI] [PubMed] [Google Scholar]
- 17.Clancy B M, Czech M P. J Biol Chem. 1990;265:12434–12443. [PubMed] [Google Scholar]
- 18.Naren A P, Di A, Cormet-Boyaka E, Boyaka P N, McGhee J R, Zhou W, Akagawa K, Fujiwara T, Thome U, Engelhardt J F, et al. J Clin Invest. 2000;105:377–386. doi: 10.1172/JCI8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hass M, Forbush B., III Annu Rev Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- 20.Prince L S, Tousson A, Marchase R B. Am J Physiol. 1993;264:C491–C498. doi: 10.1152/ajpcell.1993.264.2.C491. [DOI] [PubMed] [Google Scholar]
- 21.Chang X B, Tabcharani J A, Hou Y X, Jensen T J, Kartner N, Alon N, Hanrahan J W, Riordan J R. J Biol Chem. 1993;268:11304–11311. [PubMed] [Google Scholar]
- 22.Prince L S, Peter K, Hatton S R, Zaliauskiene L, Cotlin L F, Clancy J P, Marchase R B, Collawn J F. J Biol Chem. 1999;274:3602–3609. doi: 10.1074/jbc.274.6.3602. [DOI] [PubMed] [Google Scholar]
- 23.Smith J J, Karp P H, Welsh M J. J Clin Invest. 1994;93:307–311. doi: 10.1172/JCI117087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green S A, Spasoff A P, Coleman R A, Johnson M, Liggett S B. J Biol Chem. 1996;271:24029–24035. doi: 10.1074/jbc.271.39.24029. [DOI] [PubMed] [Google Scholar]
- 25.Rousseau G, Nantel F, Bouvier M. Mol Pharmacol. 1996;49:752–760. [PubMed] [Google Scholar]
- 26.Barak L S, Tiberi M, Freedman N J, Kwatra M M, Lefkowitz R J, Caron M G. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- 27.Clancy J P, Ruiz F E, Sorscher E J. Am J Physiol. 1999;276:C361–C369. doi: 10.1152/ajpcell.1999.276.2.C361. [DOI] [PubMed] [Google Scholar]
- 28.Hanrahan J W, Tabcharani J A, Becq F, Mathews C J, Augustinas O, Jensen T J, Chang X B, Riordan J R. Soc Gen Physiol Ser. 1995;50:125–137. [PubMed] [Google Scholar]
- 29.Hall R A, Premont R T, Chow C W, Blitzer J T, Pitcher J A, Claing A, Stoffel R H, Barak L S, Shenolikar S, Weinman E J, et al. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 30.Fouassier L, Yun C C, Fitz J G, Doctor R B. J Biol Chem. 2000;275:25039–25045. doi: 10.1074/jbc.C000092200. [DOI] [PubMed] [Google Scholar]
- 31.Cheng S H, Rich D P, Marshall J, Gregory R J, Welsh M J, Smith A E. Cell. 1991;66:1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- 32.Winter M C, Welsh M J. Nature. 1997;389:294–296. doi: 10.1038/38514. [DOI] [PubMed] [Google Scholar]
- 33.Davare M A, Avdonin V, Hall D D, Peden E M, Burette A, Weinberg R J, Horne M C, Hoshi T, Hell J W. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 34.Huang P, Lazarowski E R, Tarran R, Milgram S L, Boucher R C, Stutts M J. Proc Natl Acad Sci USA. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naren A P, Nelson D J, Xie W, Jovov B, Pevsner J, Bennett M K, Benos D J, Quick M W, Kirk K L. Nature. 1994;390:302–305. doi: 10.1038/36882. [DOI] [PubMed] [Google Scholar]
- 36.Sun F, Hug M J, Bradbury N A, Frizzell R A. J Biol Chem. 2000;275:14360–14366. doi: 10.1074/jbc.275.19.14360. [DOI] [PubMed] [Google Scholar]