Abstract
We describe an interaction between external Ca2+ ions and Shaker K channels that is important in the gating of the channels. The interaction was first detected as a partial block of inward K+ current in elevated Ca2+, beginning near −40 mV and becoming stronger at more negative voltage. Surprisingly, the time course of the block can be resolved as a rapid decay of inward current magnitude following a repolarizing step. The rapid decay of current is shown to be the result of channel block by using a two-pulse procedure that monitors the time course of gate closing. As a result of block, the decay of the tail current after repolarization is two to three times faster than gate closing. With physiological values for voltage and calcium concentration, block is readily detectable from tail time course, implying that it occurs as a normal concomitant of gate closing in Shaker. The slight voltage dependence of block from −60 to −100 mV suggests that Ca2+ is bound (with low affinity) near the outer mouth of the channel. Elevated calcium quickens the inward gating current recorded as Shaker channels are closing; this current approximately doubles in amplitude and has a faster time course and quicker rising phase. When combined, the results suggest that calcium accelerates the first step in closing of the channel gate, perhaps by changing the channel's ion-occupancy state.
Keywords: K channel‖ion channel‖gating current‖activation gate
The voltage dependence of ion channels such as the Shaker potassium channel is mainly the result of a positively charged helix built into the protein, the S4 segment. Ions in solution also affect the complex conformational changes that open and close such a channel, a topic that is less well understood. External calcium ion is known to affect the gating kinetics of many channel types (1, 2) and to block current flow in some channels even when their gates are open. Examples of block are found in the Na channel (3–5), where the block is most prominent at voltages near or negative to the resting potential, and in the cGMP-gated channels of visual photoreceptors, in which calcium block reduces channel conductance by a large factor (6). We report here that Ca2+ has a rapid blocking action on Shaker K channels, a phenomenon that has previously escaped attention. Surprisingly, the time course of the block can be resolved. We show that block leads to an acceleration of closing at physiological voltages and calcium concentrations, apparently by facilitating the first (or a very early) step in the series of conformational changes that occur as a channel's gate closes.
Methods
Early experiments (Fig. 1A) were performed at room temperature on SF9 cells expressing Shaker K channels, with inactivation in some cases removed by internal treatment with papain. The external solutions for these potassium current (IK) experiments were (in mM) 40/2 CaCl2, 48/105 NaCl (i.e., 40 Ca2+ and 48 Na+ or 2 Ca2+ and 105 Na+), 40 KCl buffered to pH 6.0 with 10 Mes-Na externally and 90 KF/30 KCl/2 MgCl2/10 EGTA/10 K Hepes (pH 7.2) internally.
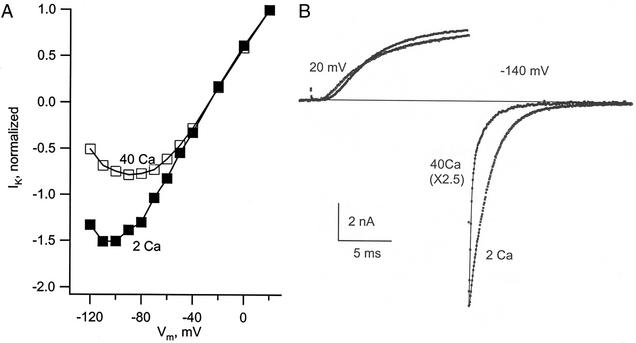
Figure 1.
Block of Shaker K channels by external Ca2+ at negative membrane voltage. (A) The curves give the current through a set of open channels in 2 and 40 mM Ca2+ as a function of membrane voltage (Vm). The channels were activated by a first 5-ms step to 40 mV, and a test step was then applied, with successive trials for the test step in the range of 20 to −120 mV. Current was measured just after the beginning of the test step, before the gates could respond to the new voltage (most gates open). Current for each solution is normalized relative to its value at 20 mV (the current at 20 mV was almost identical in the two solutions). External solutions: 2 or 40 Ca2+ with 40 K+. (B) The traces show IK of Shaker-IR channels during an activating step to 20 mV followed by a step to −140 mV, in 40 and 2 mM Ca2+. There is an obvious fast phase of decay in 40 Ca2+ caused by Ca2+ block. Solutions: 40 Ca 25 K or 2 Ca 40 K//150 K.
Later experiments (Fig. 1B and all following) used the Shaker IR channel, in which deletion of residues 6–46 removes N-type inactivation (7). These channels were expressed in HEK 293 cells, as described (8). Ionic and gating currents were recorded in whole cell configuration 27–72 h after transfection. Membrane potential was controlled by using custom-made software and hardware operated through an IBM PS-2 computer. Patch pipettes were prepared from Kimax-51 capillary tubes (Kimble, Toledo, OH), with resistance in the range of 1–2 MΩ. These low-resistance pipettes combined with a special circuit for series resistance compensation made it possible to achieve very low effective series resistance and excellent time resolution, with time constants of the capacity transient as short as 12 μs. Capacitive currents and leak were subtracted online by using a control pulse in the −140 mV to −190 mV range to generate the current signal used for subtraction.
For IK experiments with Shaker IR, the ionic compositions of the extracellular solutions (in mM) were: 40/2 Ca2+, 10 K+, 80/137 Na+, 170/159 Cl− (named 40/2 Ca 10 K); or 40/2 Ca2+, 25/40 K+, 65/107 Na+, 170/151 Cl− (named 40/2 Ca 25/40 K). The intracellular solution for IK experiments was 150 K+, 22 Cl−, 30 F−, 100 glutamate−, 1 Ca2+ 10 EGTA (named 150 K), containing 10 mM Hepes buffer, adjusted to pH 7.4. The solutions for the inward gating current (Ig) experiments were, externally (in mM), 40/4 Ca2+, 90/144 N-methylglucamine, 90/144 Cl− (named 40/4 Ca N-methylglucamine); internally, Ig experiments were 155 Cs+, 125 Cl−, 30 F−, 1 Ca2+, 10 EGTA (named 155 Cs). Solutions will be referred to as external//internal, e.g., 40 Ca 10 K//150 K. Shaker IR experiments were performed at a controlled temperature of 20°C.
Results
Calcium ions in the external medium block inward current through Shaker K channels. We first observed this in experiments with channels expressed in SF9 cells. The two curves in Fig. 1A plot the current through a set of activated K channels as a function of voltage; this curve is proportional to the current voltage relation of an open K channel. Comparing the two curves, there is clear evidence that high Ca2+ (40 mM) partially blocks inward current through the channels beginning at ≈−40 mV, and that block becomes deeper as the voltage is made more negative. In 2 mM Ca2+, the I–V relation is an almost straight line to ≈−80 mV and then shows curvature consistent with a relatively mild degree of block by Ca2+.
To better study this phenomenon, we turned to the mutant channel Shaker-IR (7), in which the inactivation ball is deleted by removing amino acid residues 6–46. This removed the overlap of two gating events that occurs in Shaker channels with intact inactivation, when closing of the activation gate is partially masked by recovery into the conducting state of inactivated channels (9). Fig. 1B shows the time course of IK as the channels open at 20 mV and the inward tail current during a second (test) step to −140 mV. We were surprised to see that in 40 mM Ca2+, the time course of block could be resolved and appears as a rapidly declining component of the IK tail. The block is much slower than expected at this high calcium concentration; a similar block in Na channels has a time course that is too fast to be resolved (5). The fast decay caused by block was followed by a slow component as the gates of the channels closed (deactivated). In 2 Ca2+, the fast component was essentially absent (Fig. 1B), making the decline of the current slow and almost exclusively the result of deactivation.
The block manifest in Fig. 1 is at high calcium concentration and very negative voltage, but calcium block can also be seen in the physiological ranges of these variables, as shown in Fig. 2. The experiment used Shaker-IR channels, and the same activation-deactivation protocol as in Fig. 1, with the experimental difference that external K+ concentration was reduced to 10 mM. Block in 40 mM Ca2+ is again clearly seen as a rapidly decaying component (half-time 195 μs) and is followed by a relatively small slow component (half-time 1.8 ms) as the channels deactivate (Fig. 2A). When recorded in 2 Ca2+, the fast component caused by block is still distinguishable, but not as large as in 40 Ca2+ (Fig. 2A).
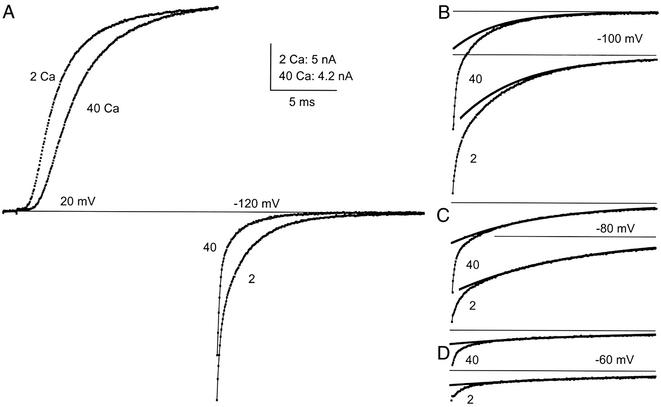
Figure 2.
Ca2+ block in 2 and 40 mM Ca2+ at physiological voltages. The pulse protocol was the same as in Fig. 1. Current is shown during the activating and repolarizing pulses in A, but only during the repolarizing pulses in B–D. The slow component of the tails was fit with a single exponential in B–D. Solutions: 2 Ca 10 K or 40 Ca 10 K//155 K.
Current tails on repolarization at three other voltages are shown in Fig. 2 B–D. In all cases, there are two easily identifiable components, and the slower one has been fit with an exponential to make clear the separation between fast and slow. From −60 to −100 mV the relative amplitude of the fast to the slow component does not change much, indicating that in this range the voltage sensitivity of block is slight. In 2 mM Ca+, for example, the ratio is ≈1 at −60 and −100 mV. Negative to −100 mV (not shown) block deepens as voltage goes negative.
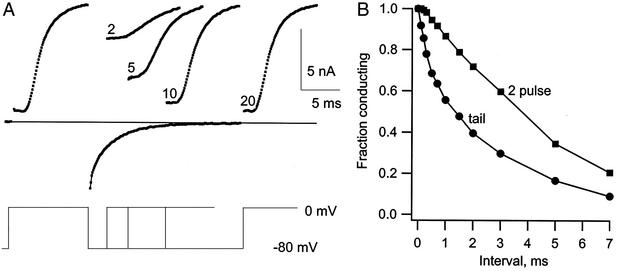
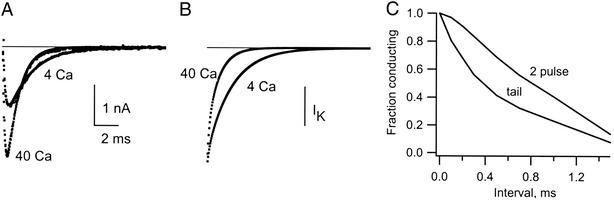
Support for the interpretation of the fast component as the result of block, and the slow component as closing of the activation gate (deactivation), is shown in Fig. 3. In this experiment, deactivation was measured with a two-pulse procedure and is compared with the time course of current decay in the tail. The two-pulse procedure (Fig. 3A) used a pulse to 0 mV to activate IK, followed by an interval of 0.1–20 ms at −80 mV. During the interval at −80 mV, some of the gates closed, and the fraction that did so could be determined from the initial current at the beginning of a test pulse back to 0 mV; the decrease of the initial current with the interpulse interval is a measure of how many channels close. After an interval of 1 ms, for example, the initial current in the test pulse is large, meaning that only a small fraction of the channels closed. The fraction still open as a function of the interval at −80 mV is compared in part B of the figure to the time course of the current tail, also at −80 mV. The tail current decays much more rapidly than the gates close as measured from the two-pulse procedure. We take the difference between the two curves as a measure of the channels that are rapidly blocked by Ca2+. On stepping back to 0 mV in the test pulse, the block is very quickly reversed. As a result, the current early in the test pulse is unaffected by the block that occurred during the interval at −80 mV. The initial current thus decays with time only because of gate closing. This comparison provides strong evidence that the fast decay of the tail current is caused by rapidly reversible block.
Figure 3.
Measurement of gate closing by a two-pulse procedure and the distinction between gate closing and block. (A) The two-pulse procedure is illustrated at left. K channels were activated by a pulse to 0 mV, and the cell was repolarized to −80 mV for 2–20 ms. (B) The fraction of the channels with gates still open after the interval at −80 mV was determined by measuring the initial current during a second pulse to 0 mV (■). ●, IK tail amplitude. The tail decays too rapidly to be explained by gate closing as measured by the two-pulse method, and the difference is the result of block by Ca2+. Solution: 40 Ca 10 K//150 K.
The time course of Ca2+ block brings to mind the properties of Ig as K channels close (10). Fig. 4A is from a cell with internal Cs+ as the only permeant ion either inside or outside. Two activating voltages are illustrated, one too negative to open many channels (−40 mV), and a higher voltage (−10 mV) that activates most channels. The activating pulse to −40 mV generates only Ig during the activating step, but the step to −10 mV causes (i) a larger Ig transient and (ii) ionic current as the channels open and (sparingly) conduct Cs+ outward. On repolarization, the current is inward and exclusively Ig because there were no permeant cations externally. Turning to the inward tail of Ig as channels close, Ig after the −40 mV activating pulse is initially large and decays quickly. After the −10 mV step, the initial amplitude of Ig is much smaller, and the time course is different; amplitude increases for ≈0.75 ms and then decays with a slow time course. For clarity, this tail is transposed upward and redrawn. The standard interpretation of this tail behavior is that the final step in the activation of channels is only slowly reversible. If the channels do not open (step to −40 mV), they return to the fully deactivated state very quickly, causing a rapidly decaying gating current that reflects the quick conformational rearrangements occurring in the channel protein. Once the channels open, however, deactivation is limited by the slowly reversible final opening (first closing) step. Judging from the initial amplitude of the gating current, opening inhibits almost all of the gating rearrangements of a channel, which must await completion of the first closing step. This step is slow and has little associated gating charge movement, resulting in a low initial amplitude of the tail (11).
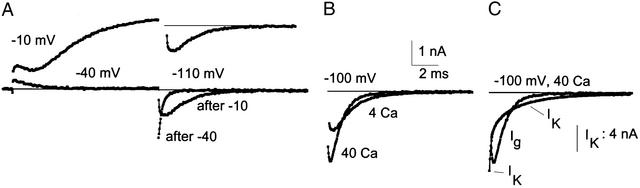
Figure 4.
Gating and ionic currents are compared. (A) In a cell containing Cs+, which permeates sparingly through K channels, Ig (−40 mV) or Ig + ICs (−10 mV) was measured during an activating pulse. Vm was then returned to −110 mV, and Ig tails were recorded (there were no permeant extracellular cations). After a step to −10 mV, the Ig tail is slow and has a rising phase. For clarity, this trace is transposed upward and repeated. (B) The Ig tail after a large activating pulse is accelerated by raising calcium from 4 to 40 mM. Charge movement in the two tails was almost the same (1% difference). (C) The rising phase of gating current compared with Ca2+ block of potassium current. The similarity in time course is striking. Solutions: Ig experiment, 40/4 Ca N-methylglucamine//155 Ca; IK experiment, 40 Ca 10 K//150 K.
The traces in Fig. 4A were recorded in 4 mM Ca2+. When Ca2+ is raised to 40 mM, tail Ig amplitude is approximately doubled and is faster. This is illustrated in Fig. 4B, where two Ig tails are superimposed, one taken with the cell in 4 Ca2+ and the other in 40 mM Ca2+. The activating pulse for both traces was to +50 mV, sufficient to open all of the channels and move all of the gating charge from resting to fully activated position. The total charge carried by the two tails as the channels close at −100 mV is, as expected, almost identical (1% different). In 40 Ca2+, the gating current rises more rapidly and to a peak that is more than twice the value in 4 Ca2+. The decay time course is also faster in 40 Ca2+; the same total charge moves in a shorter period, causing a larger current. These changes in the gating current, in particular the more rapid rise in 40 mM Ca2+ and the higher peak current, strongly suggest that high calcium speeds the first closing step, which is rate-limiting, and thus allows the subsequent steps, which generate most of the current, to proceed more quickly.
Is the more rapid rising phase of the gating current tail related to Ca2+ block? In Fig. 4C, a gating current tail is superimposed on an IK tail current (from another experiment), both recorded in 40 mM Ca2+ at −100 mV. There is a striking similarity in time course between the rising phase of the gating current and the fast, block-induced phase of IK decay. This provides strong support for the idea that calcium block of the channels in some way accelerates the first step in channel closing.
Discussion
There is incontrovertible evidence that Shaker K channels can close with 1 mM EDTA inside and out, in the absence of added divalent cations (12). The results presented here, however, show that closing is facilitated by binding of Ca2+, in physiological ionic conditions, and at the normal resting potential. The affinity for Ca2+ is quite low, and it may be that other divalents are also effective.
Two facts show that the fast component of decay in the current tails of Figs. 1 and 2 is caused by Ca2+ block. First, the fast component is larger in amplitude in high Ca2+. Second, the fast component is not due to gate closing, as is evident from the two-pulse experiment of Fig. 3. Instead, it is the result of block by Ca2+ ion, block that is very quickly removed when the voltage is returned from −80 to 0 mV.
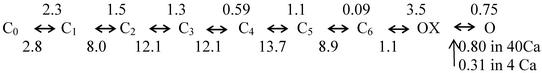
The acceleration of gate closing associated with Ca2+ block is also clear from the gating current records in Fig. 4. The amplitude of gating current is a reflection of the velocity of the gating events, and they are clearly faster in high calcium. We believe that calcium accelerates the rate-limiting first closing step, which must occur before the S4 segments can return to deactivated position. Modeling shows that the observed changes in IK and Ig tails are consistent with this idea. Fig. 5A shows gating currents measured in 4 and 40 mM Ca2+ (from Fig. 4), and, superimposed, the fits are predicted by Scheme S1, modified from Loboda and Armstrong (11).
Figure 5.
Predictions of the state model in the text for three of the experiments presented. (A) The model predictions are superimposed on experimental Ig traces taken in 4 and 40 mM Ca2+. The rate constants for the OX <> O step were fitted empirically, and all other rate constants were taken directly from Loboda and Armstrong (11) with scaling to account for temperature and other experimental differences. Beginning at the left of the reaction diagram in the text, the charge associated with each step (in electronic charges) is 0.6, 1, 1, 1.1, 0.9, 1.8, 0.7, and 0. The fits are so close that they cannot be distinguished from the data traces. (B) IK tail predictions in 4 and 40 mM Ca2+ using the rate constants from A have a strong resemblance to the experimental traces in Fig. 2 (taken in 2 and 40 mM Ca2+). (C) Predictions of the model for the experiment of Fig. 3, using the rate constants from A.
Scheme 1.
In Scheme S1, O is the conducting state. The transition to state OX (nonconducting) can occur spontaneously but is facilitated (or possibly stabilized) by the presence of Ca2+ in the pore mouth. In states O and OX, the activation gate is open. For all of the remaining states, the activation gate is closed, with the S4 units partially deactivated at the right and fully deactivated at the left (state C0). Scheme S1 was first fit to the Ig trace in 4 mM Ca2+, using the rate constants shown (in ms−1), with 0.31 ms−1 for the transition from O to OX, and then to the 40 mM Ca2+ trace using 0.8 ms−1 for O to OX (all other rate constants were unchanged). The fit is quite good in both cases; the fitted traces superimpose on (and are lost in) the data traces. The corresponding IK predictions are shown as well and look strikingly similar to the IK traces in Fig. 2. Thus, the Ig and IK changes seen on raising the Ca2+ concentration can be reproduced simply by speeding the O to OX step in the sequence above. Fig. 5B shows the predicted comparison of tail current time course and gate closing measured by the two-pulse experiment (Fig. 3). Again, the resemblance of prediction and experiment is qualitatively very close, further strengthening the idea that block by Ca2+ is an important component in the decay of the tail current.
The relative amplitudes of the fast and slow components of IK as the channels close (Fig. 2) do not change much between −60 and −100 mV. This suggests that the binding site is at the outer mouth of the pore, probably external to the selectivity filter. The events associated with calcium binding and block are not known, but the slowness of the block makes likely the involvement of a conformational change; if the binding were diffusion limited, it would be effectively instantaneous. A plausible idea is that Ca2+ speeds and/or stabilizes a conformational change in the pore's outer mouth, a change that is a necessary prelude to gate closing (see below). The fact that channels can close in the absence of Ca2+ shows that the change (O to OX in Scheme S1) can occur spontaneously, but it is apparently more favorable energetically in the presence of calcium. The nature of the conformational change is as yet unknown. It is interesting to remember that the outer pore mouth of the mutant T449C can constrict sufficiently to bind a Cd2+ ion during C-type inactivation (13). Conceivably, a similar constriction occurs when only Ca2+ and Mg2+ are present.
When the voltage is stepped negative to −100 mV, the block deepens and becomes somewhat faster. This might result from the influence of voltage on ion occupancy in the selectivity filter and the cavity internal to it (14). As described by Zhou et al. (15), there are four ion binding positions in the selectivity filter (position 1 at the outer end and position 4 at the inner), and only two are occupied simultaneously (positions 1 and 3 or 2 and 4). This picture could help explain our Ca2+ results as follows. The presence of a calcium ion in the outer mouth would be expected to repel the ions occupying the selectivity filter, thus favoring occupancy of positions 2 and 4. The presence of a cation in position 4 would in turn repel any cation occupying the cavity, making occupancy of the cavity by a cation less likely. There is evidence that occupation of the cavity by TEA+ derivatives (16, 17) or K+ (18) slows or prevents gate closing, suggesting that the cavity preferentially is empty when the gate closes. By shifting K+ ions in the filter from positions 1 and 3 to positions 2 and 4, calcium binding thus might accelerate the first closing step by making cation occupancy of the cavity less probable. This conjecture would also provide a physical explanation for the effects of Ca2+ (Fig. 4) and Ba2+ (19) on gating current generated as the channels close.
Acknowledgments
This work was supported by National Institutes of Health Grant NS 12547.
Abbreviations
- Ig
gating current
- IK
potassium current
References
- 1.Frankenhaeuser B, Hodgkin A L. J Physiol (London) 1957;137:218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 3.Woodhull A M. J Gen Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenberg C A, Bezanilla F. Biophys J. 1991;60:1499–1510. doi: 10.1016/S0006-3495(91)82185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cota G, Armstrong C M. Proc Natl Acad Sci USA. 1999;96:4154–4157. doi: 10.1073/pnas.96.7.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodoia R D, Detwiler P B. J Physiol (London) 1985;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshi T, Zagotta W N, Aldrich R W. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- 8.Jurman M E, Boland L M, Liu L Y, Yellen G. BioTechniques. 1994;17:876–881. [PubMed] [Google Scholar]
- 9.Demo S D, Yellen G. Neuron. 1991;7:743–753. doi: 10.1016/0896-6273(91)90277-7. [DOI] [PubMed] [Google Scholar]
- 10.Stefani E, Toro L, Perozo E, Bezanilla F. Biophys J. 1994;66:996–1010. doi: 10.1016/S0006-3495(94)80881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loboda A, Armstrong C M. Biophys J. 2001;81:905–916. doi: 10.1016/S0006-3495(01)75750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong K H, Armstrong C M, Miller C. Biophys J. 2001;80:2216–2220. doi: 10.1016/S0006-3495(01)76194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Jurman M, Yellen G. Neuron. 1996;16:859–867. doi: 10.1016/s0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 14.Doyle D A, Cabral J M, Pfuetzner R A, Kuo A, Gulbis J M, Cohen S L, Chait B T, MacKinnon R. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Morais-Cabral J H, Kaufman A, MacKinnon R. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong C M. J Gen Physiol. 1971;58:413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmgren M, Smith P L, Yellen G. J Gen Physiol. 1997;109:527–535. doi: 10.1085/jgp.109.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melishchuk A, Armstrong C M. Biophys J. 2001;80:2167–2175. doi: 10.1016/S0006-3495(01)76189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst R S, Roux M J, Toro L, Stefani E. Biophys J. 1997;72:77–84. doi: 10.1016/S0006-3495(97)78648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]