Abstract
The regeneration of 11-cis-retinal, the universal chromophore of the vertebrate retina, is a complex process involving photoreceptors and adjacent retinal pigment epithelial cells (RPE). 11-cis-Retinal is coupled to opsins in both rod and cone photoreceptor cells and is photoisomerized to all-trans-retinal by light. Here, we show that RPE microsomes can catalyze the reverse isomerization of 11-cis-retinol to all-trans-retinol (and 13-cis-retinol), and membrane exposure to UV light further enhances the rate of this reaction. This conversion is inhibited when 11-cis-retinol is in a complex with cellular retinaldehyde-binding protein (CRALBP), providing a clear demonstration of the protective effect of retinoid-binding proteins in retinoid processes in the eye, a function that has been long suspected but never proven. The reverse isomerization is nonenzymatic and specific to alcohol forms of retinoids, and it displays stereospecific preference for 11-cis-retinol and 13-cis-retinol but is much less efficient for 9-cis-retinol. The mechanism of reverse isomerization was investigated using stable isotope-labeled retinoids and radioactive tracers to show that this reaction occurs with the retention of configuration of the C-15 carbon of retinol through a mechanism that does not eliminate the hydroxyl group, in contrast to the enzymatic all-trans-retinol to 11-cis-retinol reaction. The activation energy for the conversion of 11-cis-retinol to all-trans-retinol is 19.5 kcal/mol, and 20.1 kcal/mol for isomerization of 13-cis-retinol to all-trans-retinol. We also demonstrate that the reverse isomerization occurs in vivo using exogenous 11-cis-retinol injected into the intravitreal space of wild type and Rpe65−/− mice, which have defective forward isomerization. This study demonstrates an uncharacterized activity of RPE microsomes that could be important in the normal flow of retinoids in the eye in vivo during dark adaptation.
The regeneration of 11-cis-retinal is critical for sustaining vision in vertebrates (for a review, see Ref. 1). The visual pigments of rod and cone photoreceptors require 11-cis-retinal, as their chromophores complex via Schiff base to opsin molecules. Upon absorption of light, 11-cis-retinal photoisomerizes to all-trans-retinal, triggering the phototransduction process through a G-protein cascade, ultimately leading to neuronal signaling (2–4). Eventually, all-trans-retinal is released by op-sin and converted back to 11-cis-retinal through a series of enzymatic steps, termed the retinoid cycle, occurring in both photoreceptor cells and adjacent RPE1 (1).
In the currently described model, the chromophore undergoes several chemical transformations. First, all-trans-retinal is released from the opsins by hydrolysis of the protonated Schiff base. It is then transported out of the rod outer segment (ROS) disks by either an ATP-binding cassette transporter (5, 6) or by simple diffusion and finally is reduced by all-trans-retinol dehydrogenase (7–10) in the reaction that appears to be the rate-limiting step in pigment regeneration (11). All-trans-retinol then diffuses into the RPE, where it is esterified to insoluble all-trans-retinyl esters by lecithin-retinol acyl transferase (LRAT) (12, 13). Either retinyl esters, retinol, or another intermediate is then employed as substrate for an isomerization reaction that converts the all-trans-retinoid to 11-cis-retinol (for a review, see Ref. 1) (Fig. 1), with the highly expressed RPE-specific protein RPE65 currently believed to play a critical role in this process (14–16). After isomerization to 11-cis-retinol (for details, see below), the retinol is oxidized to 11-cis-retinal by an NAD-specific 11-cis-retinol dehydrogenase (11-cis-RDH) and other, yet unidentified NADP-specific enzymes (17–22) (Fig. 1). Finally, 11-cis-retinal diffuses back into the ROS and cone outer segments, where it is reincorporated into opsin molecules to regenerate rhodopsin and cone pigments. 11-cis-Retinal could alternatively be produced by direct photoisomerization of all-trans-retinal with the help of retinal G-protein-coupled receptor protein in RPE and Müller cells (23, 24). The overall production of 11-cis-retinal by photoisomerization could be sufficient to drive cone pigment regeneration but insufficient to fully regenerate rod rhodopsin. Furthermore, this process would be absent in the dark-phase recovery of light-sensitive visual pigments.
Fig. 1. Conversion of all-trans-retinol in RPE.

All-trans-retinol diffuses to the RPE from ROS, and it is isomerized to 11-cis-retinol (forward reaction) and then oxidized to 11-cis-retinal by 11-cis-retinol dehydrogenases (11-cis-RDHs). Retinoids are protected by binding proteins (CRALBP and CRBP) to facilitate transport of hydrophobic substrates and also to prevent inadvertent isomerization to nonfunctional isomers such as 13-cis-retinol or unproductive conversion to all-trans-retinol (reverse isomerization). Lack of the RDH5 gene product leads to accumulation of 13-cis-retinol (22).
As described above, a crucial reaction of the retinoid cycle is the enzymatic isomerization to 11-cis-retinol. It has been proposed that the energy required for the formation of 11-cis-retinol (ΔG = ~4 kcal/mol) is provided by hydrolysis of the fatty acid chain of all-trans-retinyl esters in a reaction that involves an enzymatic nucleophilic attack at the C-11 position and alkyl cleavage of the ester, allowing free rotation around the C-11–C-12 bond and removal of the nucleophile with concomitant hydration to form 11-cis-retinol (25, 26). More recently, it has been found that apo-CRALBP greatly enhances the formation of 11-cis-retinol in vitro upon addition of all-trans-retinol to RPE microsomes (27–30). Additionally, by substituting CRBP for CRALBP, not only are all-trans-retinol and 11-cis-retinol produced, but also 13-cis-retinol in amounts that cannot be explained through spontaneous thermal isomerization (31). CRBP preferentially binds all-trans-retinol but will bind other isomers with lower affinity. These observations and others have led to the proposal that isomerization to 11-cis-retinol progresses through mass action, with binding of 11-cis-retinol to CRALBP (ΔG > 8 kcal/mol) driving the reaction, and that the transition state exists as a carbocation where electron delocalization across the polyene chain reduces the bond order, allowing isomerization to occur with the isomeric specificity of the binding protein (1, 31). Thus, this hypothetical reaction is, in part, similar to the mechanism proposed for the much more extensively studied retinol dehydratase (32).
The production of 13-cis-retinol by alternative retinoid binding proteins, demonstrates the need for stringent control of retinoids, particularly the 11-cis-retinoids that are sensitive to heat and light because of their difference in free energy from that of all-trans- and 13-cis-retinoids (ΔG = ~3–4 kcal/mol). Biochemical defects, often caused by mutations in enzymes and retinoid-binding proteins involved in the retinoid cycle, can break down the carefully protected retinoid metabolic processes, causing a number of eye diseases. Such mutations include defects in the ATP-binding cassette transporter (Stargardt disease, recessive retinitis pigmentosa, and age-related macular degeneration) (33, 34), RPE65 (Leber congenital amaurosis) (35–37), CRALBP (autosomal recessive retinitis pig-mentosa and retinitis punctata albescens) (38, 39), LRAT (early onset severe retinal dystrophy) (40), and 11-cis-RDH (fundus albipunctatus) (19, 21, 41, 42). Studies of 11-cis-RDH knockout mice (disruption of the RDH5 gene) have shown that loss of the 11-cis-retinol-specific dehydrogenase can be compensated for by other dehydrogenases within the RPE (yet to be identified), causing only minor phenotypes; however, on the biochemical level, the loss of retinoid protection and control leads to the accumulation of 11-cis-retinol/13-cis-retinol and 11-cis-retinyl esters/13-cis-retinyl esters (Fig. 1). This suggests that 11-cis-RDH is necessary to prevent perturbation of retinoid flow, and its loss results in the production of 13-cis-retinoids for which there is no known biological function (20).
Other threats to the control of retinoid flow include environmental factors, particularly damage to photoreceptors and RPE from light-induced action. Studies have shown that intense and long duration exposure to excessive light can induce damage to both the retina and RPE (43–47), with the greatest susceptibility to injury arising from wavelengths of light near the maximum absorption region of rhodopsin (~500 nm) and in the blue/UV-A region (320–450 nm) (45, 46). Evidence suggests that green light exposure is most responsible for damage incurred through chronic low level exposure, while blue/UV-A light-induced damage is incurred through short intense exposures (i.e. sun gazing, arc welding), which are able to penetrate the UV light-absorbing ability of the lens (< 400 nm) and produce damage to the retina and RPE (45, 46, 48, 49). Furthermore, it is believed that the damage is largely because of oxidation within the photoreceptor and RPE. Crockett and Lawwill showed that damage to cultured RPE from blue light (435 nm) is directly proportional to the oxygen concentration within the culture medium (44). Pautler et al. (47) demonstrated that blue light exposure to isolated RPE affects the molecular transport capabilities of the RPE and lowers their capacity for oxygen uptake. The use of antioxidants, including dimethylthiourea (50), the spin-trapping reagent phenyl-N-tert-butylnitrone (51), and, more importantly, ascorbic acid, which is naturally found in abundance in the retina but decreases during intense light exposure (52), appears to help alleviate light damage within the retina and RPE (53–56). Recently, Sparrow and Cai (57) showed that illumination of RPE with blue light triggers apoptotic processes. These cells contain the fluorophore pyridinium bisretinoid (A2E), a condensation product of all-trans-retinal and phosphoethanolamine that absorbs blue light.
Traditionally, the measure of retinal damage was performed by measuring rhodopsin or DNA levels (43). However, these measurements are typically not performed until ~2 weeks after the initial visual insult. In recent years, it has been possible to characterize the effects of light-induced retina and RPE damage at the molecular level. For example, Sun and Nathans (58) demonstrated that in the presence of all-trans-retinal, exposure of the ATP-binding cassette transporter to UV light induces its aggregation and loses activity.
In this study, we show that RPE microsomes catalyze the isomerization of 11-cis-retinol to all-trans-retinol (and 13-cis-retinol) and that the rate of isomerization is significantly augmented by exposure of RPE microsomes to UV light. However, if 11-cis-retinol is allowed to bind to CRALBP in the presence of RPE microsomes, the reaction is substantially impeded. Therefore, our results demonstrate a protective effect of retinoid-binding proteins in this process. Moreover, we demonstrated that when 11-cis-retinol is injected into the vitreal space, reverse isomerization occurs in vivo in wild type mice. Similarly, mice with defective forward isomerization (disrupted RPE65 gene (14)) also display reverse isomerization. These results suggest that, similar to in vitro conditions, the reaction is nonenzymatic in vivo as well. This study provides in depth analysis in vitro and in vivo of the novel reverse isomerization activity of cis-retinol in RPE microsomes and in the eye.
EXPERIMENTAL PROCEDURES
Materials
Bovine eyes were obtained from a local slaughterhouse (Schenk Packing Co., Inc., Stanwood, WA), and RPE microsomes were prepared as described previously (28). Additionally, mouse liver, kidney, and brain microsomes were prepared using similar methods. Microsomes were suspended in 10 mm MOPS, pH 7.0, containing 1 μm leupeptin and 1 mm dithiothreitol at a protein concentration of ~5 mg/ml as determined by the Bradford method (59). Aliquots were stored at −80 °C and were typically used within 1 month of preparation. UV treatment of microsomes was performed in 200-μl aliquots in a quartz cuvette on a ChromatoUVE transilluminator (model TM-15 from UVP Inc.) at 0 °C for 5 min unless mentioned otherwise. All experiments were carried out under dim red light.
Rpe65 Mice
All experiments involving animals were approved by the University of Washington Animal Care Committee and conformed with the recommendations of the American Veterinary Medical Association Panel on Euthanasia. All animals were maintained in complete darkness, and all manipulations were done under dim red light (Eastman Kodak Co. number 1 Safelight filter (transmittance > 560 nm)). Typically, 2–3-month-old mice were used in experiments. RPE65-deficient mice were derived from a 129/SV R1 embryonic stem cell line generated by homologous recombination with the targeting vector pPNT-RPE65 (14). This resulted in the replacement, in the targeted allele, of 1 kb of 5′-flanking region; exons 1, 2, and 3; and intervening introns of the RPE65 gene by the neo gene. The original chimeric founder was crossed to C57Bl/6 mice, and the line was maintained as a recombinant inbred line. Progeny of matings were genotyped by RPE65-specific three-primer PCR (60).
Retinoid Synthesis
[15-2H,18O]13-cis-retinol and [15-2H,18O]11-cis-retinol were synthesized based on previously published methods (31). Briefly, either 11-cis-retinal or 13-cis-retinal (1 μmol) was dissolved in 300 μl of CH3CN/H218O at a ratio of 3:1 (v/v). Next, 2 mg of p-toluenesulfonic acid or 2 mg of MOPS acid were added for 13-cis-retinol and 11-cis-retinol synthesis, respectively, and the mixture was incubated overnight at room temperature. An excess of NaBD4 was added (~5 mg), the reaction mixture was incubated on ice for 20 min and diluted with 500 μl of H2O. The retinoids were extracted with hexane (1 ml) and then purified by HPLC (HP1100; Beckman Ultrasphere-Si, 5μ; 4.6 × 250 mm; 10% ethyl acetate, 90% hexane; flow rate 1.4 ml/min). Concentrations were determined spectrophotometrically, and isotopic exchange was determined by MS (Kratos profile HV-3 direct probe).
Synthesis of Pro-R,S-[15-3H]11-cis-Retinol
To a vial containing 100 μl of 16 mm 11-cis-retinal in DMF, 500 μl of 9.6 mm [3H]NaBH4 (520 mCi/mmol; PerkinElmer Life Sciences) in DMF was added. The vial was flushed with argon, capped, and left on ice for 40 min with occasional vortexing. The reaction was stopped by adding 750 μl of H2O, and retinoids were extracted with hexane and purified by HPLC (see above).
Synthesis of [15-3H]11-cis-Retinal
To a 1.5-ml polypropylene tube containing 5 mg of MnO2 in 100 μl of DMF, 253 μl of 3.33 mm pro-R,S-[15-3H]11-cis-retinol was added. The reaction mixture was flushed with argon, capped, and placed on a mixer at room temperature for 30 min. The reaction mixture was then chilled on ice and mixed with 300 μl of ice-cold H2O. Retinoids were extracted with hexane and purified by HPLC as above.
Synthesis of Stereospecific Pro-R- or Pro-S-[15-3H]11-cis-retinol
Synthesis of pro-R- or pro-S-[15-3H]11-cis-retinol was achieved by utilizing the pro-R-specific enzyme, horse liver alcohol dehydrogenase (HLADH; Sigma) and the pro-S-specific enzyme, the purified recombinant 11-cis-RDH, as described previously (10). HLADH was first purified on a Mono Q column equilibrated with 10 mm BTP, pH 7.4, using a linear gradient from 0 to 500 mm NaCl over 60 min at a flow rate of 0.7 ml/min. The HLADH fraction (eluted at 1–3 min; 0.6 mg/ml) was dialyzed against 10 mm BTP, pH 7.4, and then used for the study.
Reverse Isomerization Assay
The reverse isomerization assay was performed using 10 μl of microsomes (RPE, liver, kidney, or brain; ~50 μg of protein) and 90 μl of 100 mm MES, pH 5.5. The reaction was initiated by the addition of 0.5 μl of 11-cis-retinol in DMF (4 mm), was incubated for 20 min at 37 °C, and was quenched by the addition of 300 μl MeOH, and the retinoids were extracted with 300 μl of hexane. The samples were vortexed for 2 min followed by centrifugation at 14,000 RPM on a microcentrifuge for phase separation. A 100-μl aliquot of the hexane extract was analyzed by HPLC (Beckman Ultrasphere-Si, 5μ, 2.0 × 250 mm, or Alltech solvent miser 5μ, 2.1 × 250 mm; 10% ethyl acetate in hexane; flow rate 0.5 ml/min), and concentrations were determined spectrophotometrically at 325 nm. The above assay conditions were used for all experiments unless mentioned otherwise. Boiled controls were obtained by boiling microsomes for at least 8 min.
Protection of 11-cis-Retinol by CRALBP from Isomerization
CRALBP (125 μg) or boiled CRALBP (~28 μm), in 90 μl of 100 mm MES, pH 6.0, was added to 10 μl of RPE microsomes treated with or without 5-min UV light. 11-cis-Retinol (0.5 μl, 4 mm in DMF) was added to a total volume of 125 μl. After incubation, the retinoids were extracted as before, or the microsomes were pelleted at 150,000 × g for 1 h at 4 °C. The supernatant and pellet were extracted as described previously.
UV and Boiling Analysis of RPE Microsomes
For the UV treatment time course, 200 μl of RPE microsomes were UV-treated. At 5, 10, 20, and 45 min of UV exposure, an aliquot of 20 μl was drawn from the cuvette and tested for reverse isomerization activity. For temperature inactivation, 200 μl of RPE microsomes were first UV-treated for 5 min, and a 20-μl aliquot was removed for testing. The remaining microsomes were then boiled for at least 8 min, and another 20-μl aliquot was removed. The cycle of UV treatment and boiling was repeated once more, followed by one more UV treatment, where each time 20 μl was removed for testing. The data are presented with S.D.
Substrate Specificity
Substrate specificity was obtained by performing time courses from 0 to 20 min using 9-cis-retinol (4 mm in DMF), 13-cis-retinol (4 mm in DMF), 11-cis-retinol (4 mm in DMF), or all-trans-retinol (4 mm in DMF). Retinols and retinals were analyzed by HPLC as described previously.
pH Profile of Forward Versus Reverse Isomerization
pH Profiles were performed using UV-treated RPE microsomes for both the reverse isomerization assay and the forward isomerization assay at pH ranges of 5.5–9.5 for the former and 6.5–9.5 for the latter, both in 0.5 pH increments. The reverse isomerization assay was performed as before, except with a 90 mm concentration of the appropriate buffer (MES for pH 5.5–6.5, BTP for pH 7.0–9.5). The forward isomerization assay used 1% bovine serum albumin, 1 mm ATP, 25 μm rCRALBP, 60 mm buffer of appropriate pH (MES for pH 6.5 and BTP for pH 7.0–9.5), 100 μg of total protein from RPE microsomes (20 μl), and 0.5 μl of all-trans-retinol (4 mm in DMF). The forward isomerization assay was incubated for 2 h at 37 °C, and retinoids were extracted and analyzed by the same method as in the reverse isomerization assay.
Determination of Activation Energies for Isomerization of 11-cis-Retinol and 13-cis-Retinol to All-transretinol
The reverse isomerization assay was performed at temperatures ranging from 6 to 53 °C in 6 °C increments. Time courses were performed at each temperature ranging from a maximum of 0–30 min in 5-min increments for lowest temperatures to 0–12 min for highest temperatures in 3-min increments. Each measurement was performed in duplicate, and the experiment was repeated for both 11-cis-retinol and 13-cis-retinol. Rates were determined from the amounts of all-trans-retinol produced, and initial rates were calculated by linear regression across all points measured for the time course. Arrhenius plots for 11-cis- and 13-cis-retinol were produced.
MS Analysis of Retinoids
The reverse isomerization assay was performed using either [15-2H,18O]11-cis-retinol or [15-2H,18O]13-cis-retinol (both 4 mm in DMF) in ~30 vials simultaneously. The hexane extract was pooled, dried down under argon, reconstituted in 200 μl of hexane, and separated on HPLC. The all-trans-retinol, 11-cis-retinol, and 13-cis-retinol fractions were collected, dried down under argon, reconstituted in 10 μl of hexane, and analyzed by MS.
Inhibition of Forward and Reverse Isomerization by Alcohols
Comparisons of inhibition between the forward isomerization assay and reverse isomerization assay were tested in a variety of conditions. To the reverse isomerization assay and forward isomerization assay, MeOH, EtOH, isopropyl alcohol, or isobutyl alcohol (2% (v/v) final concentration) was added. Retinoid analysis was done as described previously.
Inhibition of Forward and Reverse Isomerization by Protein-modifying Agents and Proteases
RPE microsomes were modified with the following reagents: acetic anhydride, N-ethylmaleimide (NEM; Sigma), phospholipase A2 (Sigma), and Pronase (Sigma).
Acetic Anhydride
RPE microsomes (50 μl, ~250 μg) were added to 40μl of 100 mm sodium borate, pH 9.0, and 10 μl of 1 m acetic anhydride in CH3CN. The mixture was allowed to incubate on ice for 10 min.
NEM
10 μl of 1 m NEM solution in 100 mm BTP, pH 7.5, was added to 90 μl of RPE microsomes (~450 μg) and incubated for 1 h at 37 °C while shaking.
Phospholipase A2
To 50 μl of RPE microsomes, ~1 μg of PLA2 was added (150 μl of total volume in 50 mm BTP, pH 7.5) and incubated for 1 h at 37 °C.
Pronase
To 50 μl of RPE microsomes, 1 μg of Pronase was added (150-μl total volume in 50 mm BTP, pH 7.5) and incubated for 1 h at 37°C.
Following each modification, the microsomes were centrifuged at 150,000 × g for 1 h at 4 °C. The microsomes were washed with 3 × 200 μl of either 100 mm MES, pH 5.5, for reverse isomerization assay or 100 mm BTP, pH 7.5, for forward isomerization and then resuspended in 50 μl of the appropriate buffer, and 10 μl was used for reverse isomerization assay, and 20 μl was used for forward isomerization assays.
Determination of the Stereoconfiguration of [15-3H]All-trans-retinol Generated by Reverse Isomerization Assay
The reverse isomerization assay was performed using either pro-R-[15-3H]11-cis-retinol (2.74 mm in DMF) or pro-S-[15-3H]11-cis-retinol (2.98 mm in DMF). The reaction was initiated by the addition of 1.0 μl of [15-3H]11-cis-retinol to the reverse isomerization assay. Retinoids were purified by HPLC, and the all-trans-retinol fractions were collected, dried down under a stream of argon, and reconstituted in DMF. The stereoconfiguration was examined with HLADH and prRDH in ROS, which are pro-R-specific enzymes with respect to the specificity of all-trans-retinol at the prochiral methylene hydroxyl group. The assay was carried out as follows (8, 10). The reaction mixture (100 μl) for the ROS assay included 83 mm sodium phosphate, pH 7.5, ROS (16 μg of rhodopsin), 1 mm dithiothreitol, 30 μm bovine serum albumin, and 600 μm NADP. The HLADH assay included BTP (62 mm with 0.05% Tween 80, pH 8.6), 21 μg of HLADH, 1 mm dithiothreitol, and 600 μm NAD. The [15-3H]all-trans-retinol (15–30 μm final concentration in the assay) generated from the reverse isomerization assay was added last to initiate the reaction. The reaction was incubated at 33 °C for 45 min (60 min for HLADH) and stopped with 400 μl of MeOH, 150 μl of 1 m NaCl, and 50 μl of 0.1 m NH2OH. After 6 min at room temperature on a mixer, 400 μl of CH2Cl2 was added. After mixing and centrifuging to separate the phases, the lower phase was removed, and the upper phase was extracted three more times with 400 μl of CH2Cl2. Radioactivity was measured in 450 μl of the upper phase by scintillation counting.
Retinoid Extraction from Mouse Eye and Analysis
All procedures were performed under dim red light as described previously (22). Retinoid analysis was performed using Beckman Ultrasphere Si 5μ (2.0 × 250 mm) and an isocratic solvent system of 0.5% ethyl acetate in hexane (v/v) for 0–10 min followed by 10% ethyl acetate in hexane from 10 to 25 min at a flow rate of 0.5 ml/min (total of 25 min). This allowed the separation of 11-cis-, 13-cis-, and all-trans-retinyl esters. Typically, two mouse eyes were used per assay and repeated two or three times. All experimental procedures related to the analyses of dissected mouse eyes and derivitization have been described in detail previously (16, 61).
Intravitreal Administration of 11-cis-Retinol
25μg of 11-cis-retinol in 1 μl of Me2SO (87.4 mM) was administered into the vitreal space employing a 10-μl syringe (model 701RN, Hamilton) with a 32-gauge × 9.37-mm needle, point style 2 (Hamilton). Mice were kept in the dark, and analysis was performed 2 weeks postintravitreal administration.
RESULTS
Protection of 11-cis-Retinol by CRALBP from Isomerization to All-transretinol
11-cis-Retinol was added to untreated RPE microsomes, and the reverse isomerization to all-trans-retinol was observed (Fig. 2A, trace a). The assay was performed at low pH to inhibit activity of LRAT, which shows maximal activity at high pH. The high level of all-trans-retinyl esters is a result of endogenous accumulation and partial esterification of exogenous retinol. To eliminate interference from endogenous retinoids in typical in vitro assays, they are destroyed by UV treatment (25). When RPE microsomes were treated with UV light for 5 min, all-trans-retinol was formed in more significant amounts (Fig. 2A, trace b), including formation of 9-cis-retinol (indicated by an asterisk). Finally, when 11-cis-retinol was added to UV-untreated (data not shown) and UV-treated microsomes in the presence of 28 μm CRALBP, isomerization to all-trans-retinol decreased dramatically (Fig. 2A, trace c, and Fig. 2B, bars a and b), suggesting that CRALBP protects 11-cis-retinol from reverse isomerization catalyzed by RPE microsomes exposure to UV light. The protective mechanisms most likely involve the binding of 11-cis-retinol into a hydrophobic pocket of CRALBP. To test this possibility, the reaction mixture was fractionated into soluble and insoluble fractions and individually analyzed. 11-cis-Retinol was present in the CRALBP-containing supernatant (Fig. 2B, bar c), and only trace amounts were found in the pellet (Fig. 2B, bar d). In contrast, when soluble and insoluble fractions were separated and analyzed in the absence of CRALBP, only trace amounts of retinoids were present in the supernatant fraction, and the pellet displayed significant formation of all-trans-retinol (data not shown). These results suggest that CRALBP plays a protective role for 11-cis-retinol, preventing its isomerization back to all-trans-retinol, as required by the flow of retinoids in physiological conditions (reviewed in Ref. 1).
Fig. 2. Protection of 11-cis-retinol by CRALBP.
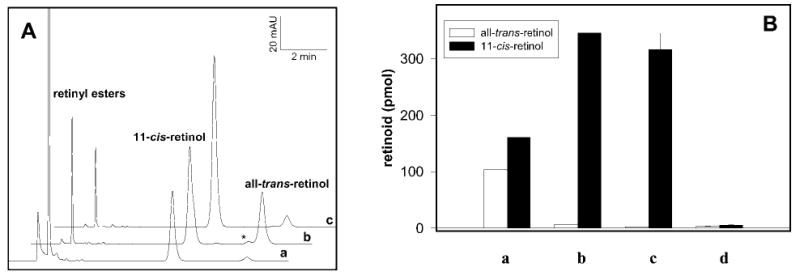
A, HPLC analysis of retinoids 20 min after the addition of 0.5 μl of 11-cis-retinol (4 mm in DMF) to untreated RPE (a), RPE microsomes treated for 5 min with UV light (b), and UV-treated microsomes with the addition of 28 μm CRALBP (c). Retinoids were extracted with hexane as described under “Experimental Procedures.” In addition to all-trans-retinol and 11-cis-retinol, a minor production of 9-cis-retinol (denoted by asterisk) after UV treatment was also observed in the absence of CRALBP, suggesting a lack of isomeric specificity for this reaction. B, the bars indicate the relative amount of all-trans-retinol and 11-cis-retinol after incubation of 11-cis-retinol in UV-treated RPE microsomes with no CRALBP (a) or the addition of ~28 μm CRALBP (b). Additional samples containing CRALBP were centrifuged to pellet membrane fractions, and the supernatant (c) and pellet (d) were analyzed for retinol content.
Evidence of Light-induced Damage to RPE Microsomes Caused by UV Light Exposure
Studies reveal that photo-oxidative damage to biological systems is dependent on the wavelength of light and intensity and the duration of light exposure (43). Therefore, to demonstrate UV damage to RPE, a time course of UV treatment was performed on RPE microsomes. After exposure of 5, 10, 20, or 45 min, an aliquot of RPE microsomes was taken and tested. Fig. 3A shows the HPLC analysis at 5, 10, 20, and 45 min (traces a, b, c, and d, respectively), demonstrating that increasing duration of UV light exposure to RPE microsomes increases the rate of isomerization of 11-cis-retinol to other isomers. Note that the formation of 13-cis-retinol (marked by an asterisk in trace d) was apparent as retinol isomers began to reach equilibrium.
Fig. 3. UV-treatment time course and temperature inactivation.
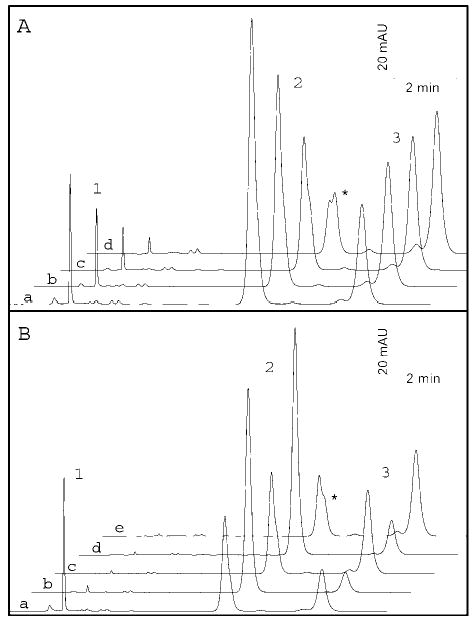
A, HPLC traces a–d show the effect of increasing UV exposure to RPE microsomes. Added 11-cis-retinol (peak 2) isomerizes to all-trans-retinol (peak 3) and then to 9-cis-retinol and 13-cis-retinol (denoted by an asterisk) as the retinoids reach equilibrium. B, temperature inactivation of UV damage to RPE microsomes. Traces a–e show the effects of alternating exposure of RPE microsomes to UV exposure (5 min) (traces a, c, and e) and boiling (8 min)(traces b and d). 13-cis-Retinol (asterisk) was also formed upon final exposure to UV treatment, suggesting accumulation of light-induced damage.
Next, to determine whether the highly reactive species created by light-induced damage could be disabled by heat, RPE microsomes were alternatively UV-treated and boiled for 8 min, this process was repeated once more, and then microsomes were subjected to a final 5-min UV treatment. After each treatment, an aliquot was removed and tested (Fig. 3B). Traces a, c, and e in Fig. 3B show the effects of UV treatment, and traces b and d show the effects of boiling for 8 min. In traces b and d, the amount of all-trans-retinol formed was greatly reduced, indicating that boiling greatly reduced isomerization catalyzed by the light-induced damage to RPE microsomes. The gradual increase in formation of all-trans-retinol and other isomers including formation of 13-cis-retinol (asterisk), following UV-treatment (traces a, c, and e), indicated that boiling for 8 min did not completely destroy all effects of light-induced damage; thus, the additional UV exposure was accumulative. These results suggest that light is responsible for generation of active species that augment the reverse isomerization.
Retinoid Substrate Specificity of UV-induced Isomerization
To test the isomeric specificity, time courses were performed to evaluate formation of all-trans-retinol from 11-cis-retinol, 13-cis-retinol, and 9-cis-retinol and the formation of 13-cis-retinol from all-trans-retinol in the presence of UV-treated RPE microsomes and boiled UV-treated RPE microsomes. The results of isomerization for retinols are shown in Fig. 4, and they indicate that the rate of all-trans-retinol formation from 11-cis-retinol and 13-cis-retinol was virtually identical, while the formation of all-trans-retinol from 9-cis-retinol was only slightly higher than the result obtained from boiled UV-treated RPE microsomes. Additionally, all-trans-retinol showed some conversion to 13-cis-retinol (Fig. 4).
Fig. 4. Substrate specificity of reverse isomerization.

Reverse isomerization was conducted in the presence of 11-cis-retinol, 13-cis-retinol, 9-cis-retinol, or all-trans-retinol for a 0–20 min time course. The top panels show chromatograms for boiled UV-treated RPE microsomes after 20 min of incubation (bottom trace) and UV-treated RPE microsomes incubated for 20 min (top trace). The peaks are identified as follows: 9-cis-retinol (peak 1), all-trans-retinol (peak 2), 11-cis-retinol (peak 3), and 13-cis-retinol (peak 4). The bottom panels compare formation of all-trans-retinol (or 13-cis-retinol in the case of the all-trans-retinol panel) in UV-treated RPE microsomes (circles) versus boiled UV-treated RPE microsomes (squares). Insets show the UV-visible spectra of selected retinoids.
Isomerization of 11-cis-retinal was measured to determine whether isomerization also occurs on the aldehyde level. The amounts of all-trans-retinal were measured by the amount of syn- and antioximes produced after treatment with NH2OH. The results showed that isomerization to all-trans-retinal was more than 5 times slower than that at the alcohol level. Additionally, no UV-dependent enhancement was observed for the isomerization of 11-cis-retinal to all-trans-retinal. Furthermore, 11-cis-retinyl palmitate was resistant to isomerization to all-trans-retinyl esters (data not shown).
Comparison of Forward Enzymatic Isomerization of All-trans-retinol with 11-cis-Retinol to Reverse Isomerization: pH Profile of Isomerization of 11-cis-Retinol
To determine whether reverse isomerization of 11-cis-retinol operated at a preferential pH, UV-treated microsomes were incubated with 11-cis-retinol at various pH values ranging from 5.5 to 9.5 in steps of 0.5 pH units (Fig. 5A). The greatest amount of all-trans-retinol formed at pH ranges of 5.5–6.0. At higher pH, the amount of all-trans-retinol formed declined sharply. Additionally, at higher pH, formation of retinyl esters increased steadily (Fig. 5B, inset). These results suggest that the low activity of the reverse isomerization at high pH could not be entirely a consequence of retinol esterification in conditions that promote higher LRAT activity. When the RPE microsomes were partially inactivated by heat treatment at 100 °C for 10 min to denature LRAT and the reverse isomerization activity was reactivated by UV light, a similar pH profile was observed (data not shown), demonstrating that the contribution of LRAT activity to affect this pH profile was minimal. For comparison, a pH profile was performed on the enzymatic isomerization of all-trans-retinol to 11-cis-retinol at a pH range of 6.5–9.5 in steps of 0.5. As expected, the peak formation of 11-cis-retinol occurs at pH 7.5, which is very close to physiological pH (Fig. 5B). Again, with higher pH, there was a corresponding increase in the formation of retinyl esters (inset). These results suggest that the two isomerization reactions may operate by different mechanisms.
Fig. 5. pH profile of reverse and forward isomerization.
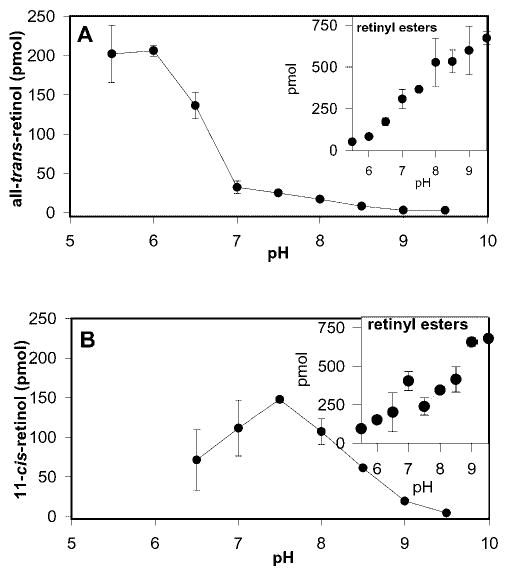
A, pH profile of reverse isomerization to all-trans-retinol. This reaction optimizes at pH 5.5–6.0 and then dramatically drops off at higher pH partially because of the increasing rate of retinyl ester formation at higher pH (inset), and the preference for isomerization to all-trans-retinol at low pH (see “Results”). B, pH profile of isomerization of all-trans-retinol to 11-cis-retinol. This enzymatic reaction optimizes at pH 7.5. The inset shows the increase in formation of retinyl esters with increasing pH.
Determination of the Activation Energy of the Isomerization of 11-cis-Retinol and 13-cis-Retinol to All-trans-retinol in UV-treated RPE Microsomes
To understand the energetics of the isomerization to all-trans-retinol, we measured the rate of isomerization of 11-cis-retinol and 13-cis-retinol to all-trans-retinol at temperatures ranging from 5 to 53 °C in 6 °C increments. At each temperature, a time course was performed to determine the rate of isomerization. Arrhenius plots were generated by graphing the logarithm of initial rates of reaction versus 1/T (Fig. 6, A and B). The activation energy was determined by a linear least-squares fitting of the data points, with the slope used in Equation 1,
Fig. 6. Arrhenius plots of isomerization of 11-cis-retinol and 13-cis-retinol to all-trans-retinol.
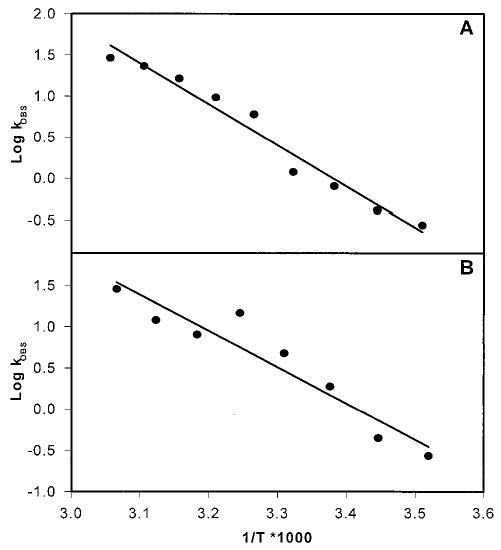
The rate of formation of all-trans-retinol was measured in duplicate at temperatures ranging from 5 to 53 °C in 6 °C increments. The log of the rate was plotted against 1/T * 1000. Linear least-squares measurement was performed, and the activation energies were calculated from the slopes and were found to be 19.5 kcal/mol for 11-cis-retinol (A) and 20.1 kcal/mol for 13-cis-retinol (B).
| (Eq. 1) |
where R is the gas constant (1.98 kcal/K·mol), and Ea is the activation energy. The calculated activation energies were found to be 19.5 kcal/mol for 11-cis-retinol (Fig. 6A) and 20.1 kcal/mol for 13-cis-retinol (Fig. 6B). These values are comparable with Ea = ~17–25 kcal/mol for all-trans-retinol to 11-cis-retinol isomerization and are in general agreement, indicating that although both the forward and reverse isomerization process proceed through different mechanisms, the intermediates could be comparable in terms of their energetics because both have the ability to lower the isomerization barrier from ~45 kcal/mol to about half (31).
Isomerization of cis-Retinols to All-trans-retinol in UV-treated Microsomes of Other Tissues
To test if isomerization could be observed in tissues other than those from bovine RPE, microsomes were produced from mouse liver, kidney, and brain, subjected to 5-min UV treatment, and measured for their ability to isomerize 11-cis-retinol, 13-cis-retinol, or 9-cis-retinol (Fig. 7A). Microsomes from these other tissues showed similar isomeric specificity as that of RPE microsomes with 9-cis-retinol, again showing little activity. Additionally, mouse liver, kidney, and brain microsomes were compared with bovine RPE microsomes for their ability to isomerize 11-cis-retinol with no UV treatment, UV treatment for 5 min, or 8-min boiling of UV-treated microsomes. Although liver, kidney, and brain microsomes showed slightly higher abilities to isomerize 11-cis-retinol to all-trans-retinol in untreated microsomes, upon UV treatment, the amount of all-trans-retinol in all microsomes increased dramatically (Fig. 7B). Again, boiling of UV-treated microsomes reduced the formation of all-trans-retinol. This provided further evidence that the reverse isomerization is nonenzymatic and significantly augmented by light treatment.
Fig. 7. Isomerization of cis-retinols to all-trans-retinol in UV-treated RPE microsomes.
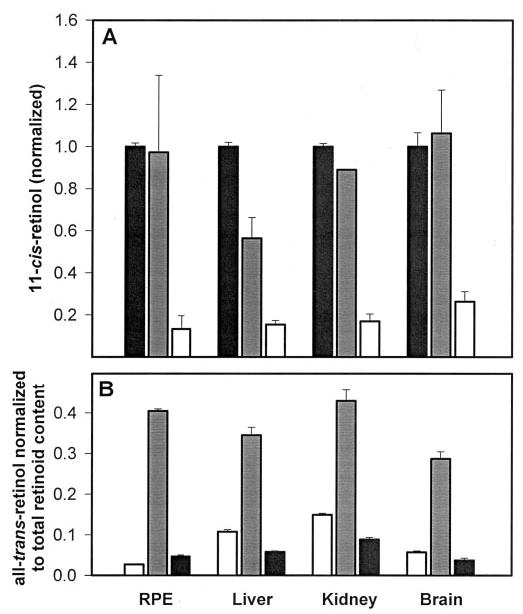
A, relative amounts of all-trans-retinol formed from cis-retinols in the presence of bovine RPE, mouse liver, kidney, and brain microsomes. Black, 11-cis-retinol; gray, 13-cis-retinol; white, 9-cis-retinol. B, relative amounts of all-trans-retinol formed from 11-cis-retinol in bovine RPE, mouse liver, kidney, and brain microsomes with no UV treatment (white), 5 min of UV treatment (gray), or 8 min of boiling following UV treatment (black). Amounts are normalized to total retinoid content measured by HPLC and spectrophotometry.
Comparison of Enzymatic Versus Nonenzymatic Isomerization by Inactivation Methods Using Alcohols, Protein-modifying Agents, and Ascorbic Acid
To demonstrate that isomerization of 11-cis-retinol to all-trans-retinol is a nonenzymatic reaction and different from the forward all-trans-retinol to 11-cis-retinol enzymatic isomerization, UV-treated RPE microsomes were treated with alcohols, protein-modifying agents, and vitamin C and measured for both isomerization reactions. Treatments were conducted with 2% methanol, 2% ethanol, 2% isopropyl alcohol, 2% isobutyl alcohol (v/v), 100 mm NEM, 100 mm acetic anhydride, 1 mg/ml phospholipase A2, and 1 mg/ml Pronase (Fig. 8A). The results show that isomerization to all-trans-retinol (gray bars) is not particularly sensitive to any of the modifications. In contrast, enzymatic isomerization to 11-cis-retinol (black bars) is significantly affected by all modifications. Furthermore, an addition of 10 mm ascorbic acid to UV-treated RPE and liver microsomes greatly reduced the amount of all-trans-retinol formed, indicating the antioxidant activity of vitamin C (Fig. 8B). The reverse isomerization was 50% inactivated by incubation of the RPE microsomes at 50 °C for 30 min, while the forward reaction was 100% inhibited by similar treatment for 5 min (data not shown). Together, these data suggest that the forward reaction is catalytic, while the reverse reaction does not involve an enzyme.
Fig. 8. Inactivation of forward and reverse isomerization by alcohols, protein-modifying reagents, and ascorbic acid.
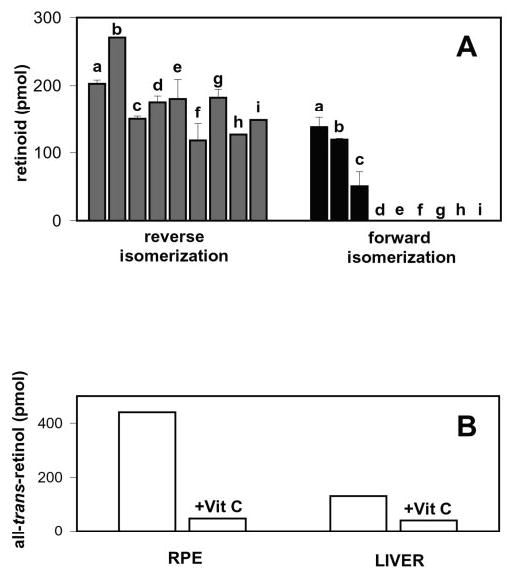
A, the effects of modifying reagents on reverse isomerization (gray) and forward isomerization (black). a, control; b, 2% MeOH; c, 2% EtOH; d, 2% isopropyl alcohol; e, 2% isobutyl alcohol; f, 100 mm NEM; g, 100 mm acetic anhydride; h, 1 mg/ml phospholipase A2; i, 1 mg/ml Pronase. UV-treated RPE microsomes were exposed to these reagents and then tested for activity. B, loss of reverse isomerization activity in the presence of 10 mm ascorbic acid in bovine RPE and liver microsomes that were UV-treated for 5 min.
MS Analysis of the Reverse Isomerization in RPE Microsomes Using [15-2H,18O]11-cis-Retinol and [15-2H,18O]13-cis-Retinol and Stereospecificity of Isomerization Using Pro-R-[15-3H]11-cis-retinol and Pro-S-[15-3H]11-cis-retinol
Reverse isomerization can proceed via retention, inversion, or mixed configuration with respect to the C-15 methylenehydroxyl group. To probe the mechanism by which isomerization of cis-retinols to all-trans-retinol occurs, retinols labeled with stable isotopes were prepared. All-trans-retinol was collected by HPLC after the addition of [15-2H,18O]11-cis-retinol or [15-2H,18O]13-cis-retinol to UV-treated RPE microsomes and analyzed by MS. Fig. 9, A and C, show MS fragmentation patterns of [15-2H,18O]13-cis-retinol and [15-2H,18O]11-cis-retinol, respectively. Note the shift to 289 (M+) because of incorporation of stable isotopes. Fig. 9, B and D, show MS fragmentation patterns of all-trans-retinol collected after isomerization of the substrates shown in Fig. 9, A and C, respectively. There was no difference in the fragmentation patterns. These results indicate that no loss of 18O occurred, suggesting that isomerization occurs because of catalysis at a site other than the alcohol functional group. Next, 11-cis-11-fluororetinol was employed as substrate (data not shown). No isomerization was observed, the same result as the forward reaction (31), suggesting that electron-withdrawing groups prevent isomerization in either direction.
Fig. 9. MS analysis and stereospecificity of isomerization of [15-2H,18O]11-cis-retinol and [15-2H,18O]13-cis-retinol to all-trans-retinol.

A and C, the fragmentation patterns of [15-2H,18O]13-cis-retinol and [15-2H,18O]11-cis-retinol, respectively, indicating the shift to 289 resulting from the presence of 2H and 18O labels. B and D, the MS fragmentation pattern of all-trans-retinol collected from reverse isomerization, indicating that there is no loss of 18O. E, retention of configuration during reverse isomerization. Pro-R-[15-3H]11-cis-retinol was used as substrate in bars 1 and 2 and bars 5 and 6, respectively. Pro-S-[15-3H]11-cis-retinol prepared using HLADH or 11-cis-RDH was used as substrate in bars 3 and 4 and bars 7 and 8, respectively. All-trans-retinol generated from reverse isomerization was collected and oxidized using ROS/NADP for bars 1, 3, 5, and 7, and with HLADH/NAD for bars 2, 4, 6, and 8.
To further determine that the alcohol functional group was not the site of reaction, the stereospecificity of isomerization was evaluated using pro-R-[15-3H]11-cis-retinol (Fig. 9E, bars 1 and 2 and bars 5 and 6, respectively) or pro-S-[15-3H]11-cis-retinol, both prepared using either HLADH or 11-cis-RDH (bars 3 and 4 and bars 7 and 8, respectively) (see “Experimental Procedures”). The all-trans-retinol produced after the addition of the above substrates to UV-treated RPE microsomes was collected and oxidized using ROS/NADP (bars 1, 3, 5, and 7) or with HLADH/NAD (bars 2, 4, 6, and 8), to stereospecifically eliminate a hydrogen atom from the C-15 position. The activity was measured by scintillation counting of the aqueous layer containing the cofactor. The results show that pro-R-[15-3H]11-cis-retinol converts to all-trans-retinol with retention of configuration by the UV-treated RPE microsomes. This was demonstrated when the isomerization product, all-trans-retinol, was oxidized by either ROS or HLADH, which have both been shown to be pro-R-specific with respect to the retinoid (22). The radioactive label was transferred to the NADP or NAD cofactor (Fig. 9E, bars 1 and 2 and bars 5 and 6). This indicates that pro-R-[15-3H]11-cis-retinol isomerizes to pro-R-[15-3H]all-trans-retinol, demonstrating that the configuration is retained. In contrast, when pro-S-[15-3H]11-cis-retinol was isomerized to all-trans-retinol and oxidized, no radiolabel was transferred to the cofactor, indicating that the pro-S-[15-3H]all-trans-retinol is formed during isomerization.
Isomerization of 11-cis-Retinol to All-trans-retinol in Vivo
To explore if reverse isomerization occurs in vivo, 11-cis-retinol was injected intravitreally into wild type mice (Rpe65+/+). In the dark, it was anticipated that 11-cis-retinol would be converted to 11-cis-retinyl esters if reverse isomerization did not occur in vivo. Compared with the control eyes, the treated eyes, which were never exposed to light, contained significantly elevated levels of all-trans-retinyl esters (Fig. 10, peak 3) and 13-cis-retinyl esters (peak 1) at the expense of 11-cis-retinol (peak 6) or 11-cis-retinyl esters (peak 2). 11-cis-Retinal bound to opsin was identified as syn- and anti-11-cis-retinal oximes (peaks 4 and 4′) and was not significantly altered in treated and untreated Rpe65+/+mice. Very similar results were obtained in four independent experiments. These results suggest that the reverse isomerization occurs in vivo.
Fig. 10. Reverse isomerization of 11-cis-retinol to all-trans-retinol in vivo.
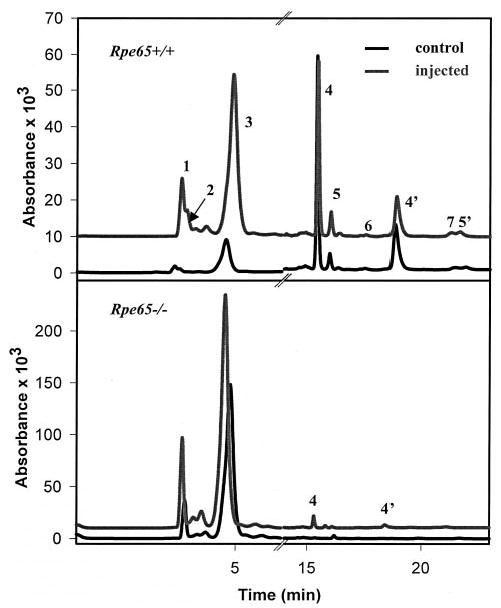
Upper panel, HPLC separation of retinoids from Rpe65+/+ and Rpe65+/+ mice administered 11-cis-retinol into the intravitreal space. Peak 1, 13-cis-retinyl esters; peak 2, 11-cis-retinyl esters; peak 3, all-trans-retinyl esters; peaks 4 and 4′, syn- and anti-11-cis-retinal oxime; peaks 5 and 5′, syn- and anti-all-trans-retinal oxime; peak 6, 11-cis-retinol; peak 7, all-trans-retinol. Lower panel, HPLC analysis of retinoids for Rpe65−/− and Rpe65−/− mice administered 11-cis-retinol into the intravitreal space.
Two possible mechanisms of reverse isomerization in vivo would involve the membrane-catalyzed isomerization observed in vitro or could isomerize through enzymatic catalysis of all-trans-retinol to 11-cis-retinol. To test between these two possibilities, 11-cis-retinol was injected intravitreally into Rpe65-/- mice, whose forward isomerization is absent (14, 16). Compared with the control eyes, significant increases in the level of all-trans-retinyl esters (Fig. 10, lower panel) and 13-cis-retinyl esters (peak 1) were observed at the expense of 11-cis-retinol, while 11-cis-retinyl esters were below detectable levels. 11-cis-Retinal bound to opsin and identified as syn- and anti-11-cis-retinal oximes (peaks 4 and 4′) was only observed in mice treated with 11-cis-retinol, suggesting that once the binding pool of 11-cis-retinoids, composed of opsin, CRALBP, and interphotoreceptor retinoid binding protein (IRBP), is saturated, the remaining amounts are converted to all-trans-retinol and esterified. Comparable results were obtained from four independent experiments. Together, these results demonstrate that reverse isomerization occurs in vivo most likely through the mechanisms extensively characterized in vitro and described above.
DISCUSSION
Protective Effect of CRALBP on the Reverse Isomerization
The importance of maintaining the integrity of 11-cis-retinal production in RPE is critical for the proper functioning of vision. Disruption of the retinoid metabolism either by genetic or environmental factors often leads to severe visual disorders (19, 21, 35–42). The roles of retinoid-binding proteins in this process, although not fully understood, are believed to be important for proper transport and protection of the retinoid moiety. Some retinoid-binding proteins are better defined than others. For example, RBP is involved in retinol transport in the blood stream (62); CRBP I functions intracellularly to partition vitamin A into a soluble form or as insoluble retinyl esters (62); and CRALBP has been initially proposed to have dual modifying effects on LRAT and 11-cis-RDH activities (63), although the interpretation of these results should be considered with caution, since these effects could be an artifact of lowering the apparent substrate concentration and the higher affinity of CRALBP for the aldehyde than alcohol of 11-cis-isomer, respectively. The most profound stimulatory effect of CRALBP is on the rate of isomerization reaction through mass action (31). In this study, we investigated the effects of RPE microsomes on the isomerization of 11-cis-retinol and 13-cis-retinol to all-trans-retinol. These reactions were further augmented by UV treatment of RPE membranes in vitro. When CRALBP is added to UV-treated microsomes, 11-cis-retinol is protected from isomerization by the resulting UV-induced products, probably shielded within a hydrophobic pocket in the core of the CRALBP protein (Fig. 2). The hypothesis of a hydrophobic pocket to protect retinoids is based on analogy to the crystal structures of CRBP and RBP (reviewed in Ref. 62), since the high resolution structure of CRALBP is not yet known.
Only a limited number of studies have shown direct protective roles for retinoid-binding proteins. One investigation of UV light exposure on hairless mice found that it greatly reduced the concentrations of retinyl esters, although all-trans-retinol was largely unaffected. This suggested that retinoid-binding proteins such as CRBP I protect all-trans-retinol from the damaging effects of UV light (64, 65). Although more recent work has suggested that CRBP’s primary role in UV-mediated damage is more related to retinoid mobilization in response to the light-induced injury (66), other studies of UV exposure to human skin and to Gecko lens further support the protective role of CREB I (64, 67). In the case of IRBP, Crouch et al. (68) showed that 11-cis-retinol could be protected from both oxidation and isomerization if bound to IRBP. Our studies add to these observations, showing the important protective role of CRALBP for proper flow of retinoids in RPE microsomes.
Chemistry of the Reverse Isomerization
We performed several experiments to demonstrate that the isomerization of 11-cis-retinol in RPE microsomes is not an enzymatic process that is further augmented by a by-product of UV treatment. The extended duration of UV treatment accelerates the formation of all-trans-retinol and 13-cis-retinol (Fig. 3A), and by alternating UV exposure and boiling, reverse isomerization activity is gained and diminished, although not completely in the boiling stage, allowing cumulative damage during UV treatment. This is documented by the formation of 13-cis-retinol in the third cycle (Fig. 3B). Similar activity is found when microsomes were produced from the liver, kidney, and brain of mouse (Fig. 7). Chemical modifications that either reduced or eliminated the enzymatic isomerization of all-trans-retinol to 11-cis-retinol had little effect on the nonenzymatic reverse isomerization, and the addition of 10 mm ascorbic acid to UV-treated RPE or liver microsomes quenched isomerization (Fig. 8B). This supports the previous studies on the protective effects of ascorbic acid from light-induced damage in vivo (69).
Substrate specificity experiments showed that while 11-cis-retinol and 13-cis-retinol isomerized to all-trans-retinol at similar rates, 9-cis-retinol was less affected, and all-trans-retinol isomerized to 13-cis-retinol at a slower rate than expected. Experimental calculation of the activation energy of isomerization of 11-cis-retinol and 13-cis-retinol to all-trans-retinol confirmed their similarity with 19.5 kcal/mol and 20.1 kcal/mol, respectively. These values were also in good agreement with the 17–25 kcal/mol calculated for the enzymatic isomerization to 11-cis-retinol (31). Additionally, MS analysis and stereospecificity experiments indicate that the hydroxyl group is not disturbed during reverse isomerization (Fig. 9). These results eliminate a number of possible mechanisms by which isomerization could occur, most notably dehydration and formation of retinyl carbocation.
The formation of a carbocation by the addition of an electrophile (proton) to the polyene chain might be a more likely mechanism of isomerization that would preserve the OH group. This mechanism is favorable at low pH, as we observed experimentally. 11-cis-Retinol and 13-cis-retinol are preferable possibly because of nonlinear electron distribution along the polyene chain. These reactions described here are different from earlier studies of the much slower cis-trans isomerization of retinals in the presence of phosphatidylethanolamine (70), crude tissue extracts (71), lipid dispersion (72), or ROS (73).
Alternatively, the mechanism may involve thio radicals, as demonstrated for cis-trans isomerization of polyunsaturated fatty acid residues in phospholipids (74). Although we observed only a ~50% reduction in reverse isomerization when membranes were NEM-treated, the UV-treated RPE membranes were active even after a few days of storage at 4 °C (data not shown). These findings could be explained by a stable intermediate deep in the membranes that is not accessible for NEM modification. All together, we favor this mechanism less than electrophilic addition to the polyene chain, because thio radicals are predicted to be very unstable.
Implication of Reverse Isomerization to Biochemical Studies of Forward Isomerization
Our studies also provide new insights into isomerization in vitro for the forward reaction. Over the years, the isomerization of all-trans-retinol to 11-cis-retinol in vitro was hampered by exceedingly low production of 11-cis-retinol (25, 75, 76). A major enhancement of isomerization was observed in just the last few years when the retinoid-binding proteins CRALBP and/or CRBP were included in the assays (1, 27–29, 31). The explanation for low activity in the earlier studies could be accounted for by two processes: 1) UV-treated membranes were used that promoted reverse isomerization, and 2) the lack of retinoid-binding proteins pooled thermodynamically unfavorable reaction products (28, 29, 31).
Implication of Reverse Isomerization in Vivo
In wild type mice, reverse isomerization also occurs in vivo. When 11-cis-retinol was injected into the eye of dark-adapted mice, more than 95% was converted to the all-trans-isomer. Due to very high activity of LRAT in the RPE, retinols are stored in the form of fatty acid esters (reviewed in Ref. 1). This observation explains why mice have a very low pool of 11-cis-retinyl esters and lends additional support to the idea that the different pools of retinoid in the eye are in chemical and thermodynamic equilibria rather than driven by energetically driving coupled reactions. From several potential mechanisms of reverse isomerization, two appear to have the highest probability. One involves reverse isomerization through the enzymatic steps present in the RPE that normally produce 11-cis-retinol from all-trans-retinol but run in reverse. The second is that the 11-cis-retinol conversion to all-trans-retinol is a result of the reverse isomerization similar to that characterized in the study using in vitro assays. The strong support of a nonenzymatic reaction comes from the analysis of Rpe65−/− mice, which have a defective forward isomerization reaction (14, 16). Almost complete isomerization of 11-cis-retinol injected into the eye in the dark was observed, suggesting that the forward reaction is not essential for the reverse isomerization. Importantly, higher levels of 13-cis-retinyl esters were observed in treated Rpe65+/+ and Rpe65−/− mice, showing resemblance to the in vitro reverse isomerization.
Although a mechanistic correlation between the reverse isomerization in vivo and in vitro cannot be confirmed, a number of facts suggest that they are similar. First, both reactions produce all-trans, and subsequently 13-cis isomers2 from 11-cis-retinol. Second, both reactions appear to proceed independently of the enzymatic steps for all-trans-retinol to 11-cis-retinol isomerization, because the in vitro reaction is nonenzymatic, and the in vivo reaction does not require RPE65. Third, previous studies have shown that activation of the only functional group on 11-cis-retinol (and all-trans-retinol) often causes rearrangements resulting in the formation of anhydroretinol or retroretinols (33). The carbocation mechanism proposed for the enzymatic forward isomerization is plausible because the transition state can be stabilized in the active site of the enzyme, preventing anhydroretinol formation. However, in our in vivo retinoid analysis of wild type and RPE65−/− mice, no anhydroretinol or retroretinol was observed. This suggested that in vivo the hydroxyl group of 11-cis-retinol was not involved in the reverse isomerization process.
In summary, we show that exposure of RPE microsomes to UV light generates products that catalyze the isomerization of 11-cis-retinol to all-trans-retinol when 11-cis-retinol is unprotected. This reaction is offset physiologically by the requirement to convert all-trans-retinol to 11-cis-retinol. This reverse isomerization is inhibited when 11-cis-retinol is protected in a complex with CRALBP. The reverse isomerization is nonenzymatic and specific to alcohol forms of retinoids and displays a stereospecific preference. It occurs with the retention of configuration at the methylenehydroxyl C-15 carbon of retinol through a mechanism that does not eliminate the hydroxyl group; in contrast, the forward reaction undergoes a mechanism that eliminates the hydroxyl group. The Arrhenius plots show that the activation energy was found to be 19.5 kcal/mol for 11-cis-retinol to all-trans-retinol and 20.1 kcal/mol for the 13-cis-retinol to all-trans-retinol isomerization reaction, suggesting that an energetically similar intermediate is formed as the proposed carbocation for the forward reaction. Finally, the reverse isomerization occurs in vivo, with and without functional enzymes of forward isomerization.
Acknowledgments
We thank Drs. Rosalie K. Crouch, David S. Papermaster, and Vladimir Kuksa for comments on the manuscript; Dr. Michael Redmond for Rpe65 mice; Dr. Angel Rodriguez de Lera for 11-cis-11-fluororetinal; and Dr. Martin Sadilek for assistance with MS analysis.
Footnotes
This work was supported by National Institutes of Health Grants EY07031 (vision training grant; to J. K. M.), EY09339 and EY66-3988 (Research to Prevent Blindness, Inc. (RPB) to the Department of Ophthalmology at the University of Washington), by the Ruth and Milton Steinbach Fund, by the Alcon Research Institute, and by the E. K. Bishop Foundation.
The abbreviations used are: RPE, retinal pigment epithelial cell(s); CRALBP, cellular retinaldehyde-binding protein; CRBP, cellular retinol-binding protein; LRAT, lecithin-retinol acyltransferase; MES, 4-morpholineethanesulfonic acid; MOPS, 3-[N-morpholino]propanesulfonic acid; MS, mass spectrometry; NEM, N-ethylmaleimide; ROS, rod outer segments; RDH, retinol dehydrogenase; HPLC, high pressure liquid chromatography; BTP, 1,3-bis[tris(hydroxymethyl)-methyl-amino]propane); DMF, N,N-dimethylformamide.
It is worth speculating that formation of 13-cis-retinoids in RDH5 knockout mice (23) could be a result of the lower affinity of CRALBP toward 11-cis-retinol as compared with 11-cis-retinal. Thus, CRALBP protects against reverse isomerization in native conditions, but this process is modified in conditions of lower oxidation power in RDH5 knockout mice, leading to production of 13-cis-retinoids (Fig. 1).
References
- 1.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Prog Retin Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 2.Polans A, Baehr W, Palczewski K. Trends Neurosci. 1996;19:547–554. doi: 10.1016/s0166-2236(96)10059-x. [DOI] [PubMed] [Google Scholar]
- 3.Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Physiol Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- 4.Lamb TD, Pugh EN., Jr Trends Neurosci. 1992;15:291–298. doi: 10.1016/0166-2236(92)90079-n. [DOI] [PubMed] [Google Scholar]
- 5.Sun H, Molday RS, Nathans J. J Biol Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- 6.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 7.Haeseleer F, Huang J, Lebioda L, Saari JC, Palczewski K. J Biol Chem. 1998;273:21790–21799. doi: 10.1074/jbc.273.34.21790. [DOI] [PubMed] [Google Scholar]
- 8.Palczewski K, Jäger S, Buczylko J, Crouch RK, Bredberg DL, Hofmann KP, Asson-Batres MA, Saari JC. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- 9.Rattner A, Smallwood PM, Nathans J. J Biol Chem. 2000;275:11034–11043. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- 10.Jang GF, McBee JK, Alekseev AM, Haeseleer F, Palczewski K. J Biol Chem. 2000;275:28128–28138. doi: 10.1074/jbc.M004488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palczewski K, Van Hooser JP, Garwin GG, Chen J, Liou GI, Saari JC. Biochemistry. 1999;38:12012–12019. doi: 10.1021/bi990504d. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D. J Biol Chem. 1999;274:3834–3841. doi: 10.1074/jbc.274.6.3834. [DOI] [PubMed] [Google Scholar]
- 13.Mondal MS, Ruiz A, Bok D, Rando RR. Biochemistry. 2000;39:5215–5220. doi: 10.1021/bi9929554. [DOI] [PubMed] [Google Scholar]
- 14.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 15.Tsilou E, Hamel CP, Yu S, Redmond TM. Arch Biochem Biophys. 1997;346:21–27. doi: 10.1006/abbi.1997.0276. [DOI] [PubMed] [Google Scholar]
- 16.Van Hooser JP, Aleman TS, He YG, Cideciyan AV, Kuksa V, Pittler SJ, Stone EM, Jacobson SG, Palczewski K. Proc Natl Acad Sci U S A. 2000;97:8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon A, Hellman U, Wernstedt C, Eriksson U. J Biol Chem. 1995;270:1107–1112. [PubMed] [Google Scholar]
- 18.Driessen CA, Janssen BP, Winkens HJ, van Vugt AH, de Leeuw TL, Janssen JJ. Invest Ophthalmol Vis Sci. 1995;36:1988–1996. [PubMed] [Google Scholar]
- 19.Yamamoto H, Simon A, Eriksson U, Harris E, Berson EL, Dryja TP. Nat Genet. 1999;22:188–191. doi: 10.1038/9707. [DOI] [PubMed] [Google Scholar]
- 20.Driessen CA, Winkens HJ, Hoffmann K, Kuhlmann LD, Janssen BP, Van Vugt AH, Van Hooser JP, Wieringa BE, Deutman AF, Palczewski K, Ruether K, Janssen JJ. Mol Cell Biol. 2000;20:4275–4287. doi: 10.1128/mcb.20.12.4275-4287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cideciyan AV, Haeseleer F, Fariss RN, Aleman TS, Jang GF, Verlinde CL, Marmor MF, Jacobson SG, Palczewski K. Vis Neurosci. 2000;17:667–678. doi: 10.1017/s0952523800175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang GF, Van Hooser JP, Kuksa V, McBee JK, He YG, Janssen JJ, Driessen CA, Palczewski K. J Biol Chem. 2001;276:32456–32465. doi: 10.1074/jbc.M104949200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao L, Shen D, Pandey S, Hao W, Rich KA, Fong HK. Mol Vis. 1998;4:25. [PubMed] [Google Scholar]
- 24.Hao W, Chen P, Fong HK. Methods Enzymol. 2000;316:413–422. doi: 10.1016/s0076-6879(00)16739-4. [DOI] [PubMed] [Google Scholar]
- 25.Deigner PS, Law WC, Canada FJ, Rando RR. Science. 1989;244:968–971. doi: 10.1126/science.2727688. [DOI] [PubMed] [Google Scholar]
- 26.Rando RR. Chem Biol. 1996;3:255–262. doi: 10.1016/s1074-5521(96)90105-2. [DOI] [PubMed] [Google Scholar]
- 27.Winston A, Rando RR. Biochemistry. 1998;37:2044–2050. doi: 10.1021/bi971908d. [DOI] [PubMed] [Google Scholar]
- 28.Stecher H, Gelb MH, Saari JC, Palczewski K. J Biol Chem. 1999;274:8577–8585. doi: 10.1074/jbc.274.13.8577. [DOI] [PubMed] [Google Scholar]
- 29.Stecher H, Prezhdo O, Das J, Crouch RK, Palczewski K. Biochemistry. 1999;38:13542–13550. doi: 10.1021/bi9913294. [DOI] [PubMed] [Google Scholar]
- 30.Stecher H, Palczewski K. Methods Enzymol. 2000;316:330–344. doi: 10.1016/s0076-6879(00)16733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBee JK, Kuksa V, Alvarez R, de Lera AR, Prezhdo O, Haeseleer F, Sokal I, Palczewski K. Biochemistry. 2000;39:11370–11380. doi: 10.1021/bi001061c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pakhomova S, Kobayashi M, Buck J, Newcomer ME. Nat Struct Biol. 2001;8:447–451. doi: 10.1038/87617. [DOI] [PubMed] [Google Scholar]
- 33.Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 34.Allikmets R. Nat Genet. 1997;17:122. doi: 10.1038/ng0997-122a. [DOI] [PubMed] [Google Scholar]
- 35.Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Nat Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 36.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Proc Natl Acad Sci U S A. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 38.Morimura H, Berson EL, Dryja TP. Invest Ophthalmol Vis Sci. 1999;40:1000–1004. [PubMed] [Google Scholar]
- 39.Maw MA, Kennedy B, Knight A, Bridges R, Roth KE, Mani EJ, Mukkadan JK, Nancarrow D, Crabb JW, Denton MJ. Nat Genet. 1997;17:198–200. doi: 10.1038/ng1097-198. [DOI] [PubMed] [Google Scholar]
- 40.Thompson DA, Li Y, McHenry CL, Carlson TJ, Ding X, Sieving PA, Apfelstedt-Sylla E, Gal A. Nat Genet. 2001;28:123–124. doi: 10.1038/88828. [DOI] [PubMed] [Google Scholar]
- 41.Kuroiwa S, Kikuchi T, Yoshimura N. Am J Ophthalmol. 2000;130:672–675. doi: 10.1016/s0002-9394(00)00765-0. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Fernandez F, Kurz D, Bao Y, Newman S, Conway BP, Young JE, Han DP, Khani SC. Mol Vis. 1999;5:41. [PubMed] [Google Scholar]
- 43.Organisciak DT, Winkler BS. Prog Retina Eye Res. 1994;13:1–29. [Google Scholar]
- 44.Crockett RS, Lawwill T. Curr Eye Res. 1984;3:209–215. doi: 10.3109/02713688408997202. [DOI] [PubMed] [Google Scholar]
- 45.Rapp LM, Tolman BL, Dhindsa HS. Invest Ophthalmol Vis Sci. 1990;31:1186–1190. [PubMed] [Google Scholar]
- 46.Rapp LM, Smith SC. Invest Ophthalmol Vis Sci. 1992;33:3367–3377. [PubMed] [Google Scholar]
- 47.Pautler EL, Morita M, Beezley D. Photochem Photobiol. 1990;51:599–605. doi: 10.1111/j.1751-1097.1990.tb01972.x. [DOI] [PubMed] [Google Scholar]
- 48.Ham WT, Jr, Mueller HA, Sliney DH. Nature. 1976;260:153–155. doi: 10.1038/260153a0. [DOI] [PubMed] [Google Scholar]
- 49.Gorgels TG, van Norren D. Invest Ophthalmol Vis Sci. 1995;36:851–863. [PubMed] [Google Scholar]
- 50.Organisciak DT, Darrow RM, Jiang YI, Marak GE, Blanks JC. Invest Ophthalmol Vis Sci. 1992;33:1599–1609. [PubMed] [Google Scholar]
- 51.Ranchon I, Chen S, Alvarez K, Anderson RE. Invest Ophthalmol Vis Sci. 2001;42:1375–1379. [PubMed] [Google Scholar]
- 52.Organisciak DT, Wang HM, Kou AL. Curr Eye Res. 1984;3:257–267. doi: 10.3109/02713688408997208. [DOI] [PubMed] [Google Scholar]
- 53.Organisciak DT, Wang HM, Li ZY, Tso MO. Invest Ophthalmol Vis Sci. 1985;26:1580–1588. [PubMed] [Google Scholar]
- 54.Organisciak DT, Bicknell IR, Darrow RM. Curr Eye Res. 1992;11:231–241. doi: 10.3109/02713689209001774. [DOI] [PubMed] [Google Scholar]
- 55.Organisciak DT, Jiang YL, Wang HM, Bicknell I. Invest Ophthalmol Vis Sci. 1990;31:1195–1202. [PubMed] [Google Scholar]
- 56.Li ZY, Tso MO, Wang HM, Organisciak DT. Invest Ophthalmol Vis Sci. 1985;26:1589–1598. [PubMed] [Google Scholar]
- 57.Sparrow JR, Cai B. Invest Ophthalmol Vis Sci. 2001;42:1356–1362. [PubMed] [Google Scholar]
- 58.Sun H, Nathans J. J Biol Chem. 2001;276:11766–11774. doi: 10.1074/jbc.M010152200. [DOI] [PubMed] [Google Scholar]
- 59.Bradford MM. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 60.Redmond TM, Hamel CP. Methods Enzymol. 2000;316:705–724. doi: 10.1016/s0076-6879(00)16758-8. [DOI] [PubMed] [Google Scholar]
- 61.Van Hooser JP, Garwin GG, Saari JC. Methods Enzymol. 2000;316:565–575. doi: 10.1016/s0076-6879(00)16750-3. [DOI] [PubMed] [Google Scholar]
- 62.Noy N. Biochem J. 2000;348:481–495. [PMC free article] [PubMed] [Google Scholar]
- 63.Saari JC, Bredberg DL, Noy N. Biochemistry. 1994;33:3106–3112. doi: 10.1021/bi00176a045. [DOI] [PubMed] [Google Scholar]
- 64.Tang G, Webb AR, Russell RM, Holick MF. Photodermatol Photoimmunol Photomed. 1994;10:1–7. [PubMed] [Google Scholar]
- 65.Sorg O, Tran C, Carraux P, Didierjean L, Saurat J. Dermatology. 1999;199:302–307. doi: 10.1159/000018279. [DOI] [PubMed] [Google Scholar]
- 66.Tran C, Sorg O, Carraux P, Didierjean L, Saurat JH. Photochem Photobiol. 2001;73:425–431. doi: 10.1562/0031-8655(2001)073<0425:tdorct>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 67.Roll B, van Boekel MA, Amons R, de Jong WW. Biochem Biophys Res Commun. 1995;217:452–458. doi: 10.1006/bbrc.1995.2797. [DOI] [PubMed] [Google Scholar]
- 68.Crouch RK, Hazard ES, Lind T, Wiggert B, Chader G, Corson DW. Photochem Photobiol. 1992;56:251–255. doi: 10.1111/j.1751-1097.1992.tb02154.x. [DOI] [PubMed] [Google Scholar]
- 69.Organisciak DT, Wang HM, Noell WK. Prog Clin Biol Res. 1987;247:455–468. [PubMed] [Google Scholar]
- 70.Groenendijk GW, Jacobs CW, Bonting SL, Daemen FJ. Eur J Biochem. 1980;106:119–128. doi: 10.1111/j.1432-1033.1980.tb06002.x. [DOI] [PubMed] [Google Scholar]
- 71.Sack RA, Seltzer S. Vision Res. 1978;18:423–426. doi: 10.1016/0042-6989(78)90052-4. [DOI] [PubMed] [Google Scholar]
- 72.Fulton BS, Rando RR. Biochemistry. 1987;26:110–114. doi: 10.1021/bi00375a016. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu T, Ishiguro S, Tamai M. J Biochem (Tokyo) 1998;123:953–958. doi: 10.1093/oxfordjournals.jbchem.a022030. [DOI] [PubMed] [Google Scholar]
- 74.Ferreri C, Costantio C, Perrotta L, Landi L, Mulazzani QG, Chargilialoglu C. J Am Chem Soc. 2001;123:4459–4468. doi: 10.1021/ja0040969. [DOI] [PubMed] [Google Scholar]
- 75.Bernstein PS, Law WC, Rando RR. Proc Natl Acad Sci U S A. 1987;84:1849–1853. doi: 10.1073/pnas.84.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Law WC, Rando RR. Biochemistry. 1988;27:4147–4152. doi: 10.1021/bi00411a037. [DOI] [PubMed] [Google Scholar]


