Abstract
Background: Forced-air warming is sometimes unable to maintain perioperative normothermia. We therefore compared heat transfer, regional heat distribution, and core rewarming of forced-air warming with a novel circulating-water garment.
Methods: Nine volunteers were each evaluated on two randomly ordered study days. They were anesthetized and cooled to a core temperature near 34°C. The volunteers were subsequently warmed for 2.5 hours with either a circulating-water garment or forced-air cover. Overall, heat balance was determined from the difference between cutaneous heat loss (thermal flux transducers) and metabolic heat production (oxygen consumption). Average arm and leg (peripheral) tissue temperatures were determined from 18 intramuscular needle thermocouples, 15 skin thermal flux transducers, and “deep” arm and foot thermometers.
Results: Heat production (≈ 60 kcal/h) and loss (≈45 kcal/h) were similar with each treatment before warming. The increase in heat transfer across anterior portions of the skin surface was similar with each warming system (≈65 kcal/h). Forced-air warming had no effect on posterior heat transfer whereas circulating-water transferred 21 ± 9 kcal/h through the posterior skin surface after a half hour of warming. Over 2.5 h, circulating-water thus increased body heat content 56% more than forced air. Core temperatures thus increased faster than with circulating water than forced air, especially during the first hour, with the result that core temperature was 1.1 ± 0.7°C greater after 2.5 h (P < 0.001). Peripheral tissue heat content increased twice as much as core heat content with each device, but the core-to-peripheral tissue temperature gradient remained positive throughout the study.
Conclusions: The circulating-water system transferred more heat than forced air, with the difference resulting largely from posterior heating. Circulating water rewarmed patients 0.4°C/h faster than forced air. A substantial peripheral-to-core tissue-temperature gradient with each device indicated that peripheral tissues insulated the core, thus slowing heat transfer.
Introduction
Perioperative hypothermia is common and results from anesthetic-induced inhibition of thermoregulatory control combined with cold exposure.1 Even mild perioperative hypothermia causes numerous complications including morbid myocardial events,2 surgical wound infections,3 coagulopathy,4 and prolonged hospitalization.3 Consequently, most anesthesiologists attempt to maintain intraoperative normothermia (i.e., core temperature ≈37°C).
Forced-air warming transfers large amounts of heat across the skin surface5 and has been proven effective in most operations.6,7 However, there are some clinical situations in which forced-air warming cannot maintain normothermia. Common examples include liver transplantation, off-pump coronary artery bypass, polytrauma, and major abdominal surgery conducted in lithotomy position.8 The difficulty in each case is inadequate surface area for effective cutaneous heat transfer.
Because forced-air warming is sometimes insufficient, a more effective warming system is needed. A recently developed alternative (Allon, Medical Thermoregulation Equipment [MTRE], Or Akiva, Israel) circulates warm water through a special garment (circulating-water garment).9,10 The conductive-heating garment is segmented which allows clinicians to cover relatively large surface areas. We therefore measured cutaneous heat transfer, body heat distribution, and core rewarming rates with a forced-air cover or a circulating-water and compared them.
The body can be roughly divided into core (trunk and head) and peripheral (extremities) thermal compartments.11 A limitation of cutaneous warming systems is that heat is transferred into the peripheral thermal compartment. Depending on vasomotor status, flow of heat to the core, and therefore core warming, can be substantially delayed.12 The primary reason is that peripheral tissue temperatures are typically 2°C to 4°C less than core temperature.11 Heat transferred into peripheral tissues therefore cannot flow into the core without violating the Second Law of Thermodynamics. We therefore evaluated flow of heat between the peripheral and core thermal compartments at two different cutaneous warming rates. Specifically, we determined the extent to which peripheral tissues need to be heated before core warming becomes effective and the relation between peripheral and core tissue temperatures.
Methods
With approval of the Institutional Review Board at Washington University and informed consent, we studied nine healthy volunteers (five men, four women). Each was evaluated on two randomly assigned study days, separated by at least 48 hours. None was obese, pregnant, taking any medication, or had a history of infection, recent fever, diabetes, problems with general anesthesia, or a neuromuscular disease. The subjects were minimally clothed and reclined on a padded table in the Anesthesia Clinical Research Area during the study. Ambient temperature was maintained at ≈21°C.
Protocol
Studies started at approximately 9:30 AM, and volunteers fasted during the 8 hours preceding each study. A catheter was inserted into the right antecubital vein. Anesthesia was induced by intravenous administration of propofol (3-5 mg/kg) and vecuronium bromide (0.1 mg/kg). The volunteers' tracheas were intubated, and mechanical ventilation was adjusted to maintain end-tidal PCO2 near 35 mmHg. Anesthesia was maintained with desflurane (5.5%) in 40% oxygen and air during the cooling period and with propofol in 40% oxygen and air during the warming period. An infusion of vecuronium was adjusted to maintain one mechanical twitch in response to supramaximal train-of-four electrical stimulation of the ulnar nerve at the wrist.
The volunteers were actively cooled to an esophageal temperature of 34°C with a forced-air cover set to 10°C (Polar Air, Augustine Medical, Inc., Eden Prairie, MN). The desflurane concentration was decreased, if necessary, to trigger arterio-venous shunt vasoconstriction (see Measurements below); constriction was maintained for 30 minutes (control period). Desflurane was then discontinued and anesthesia was maintained by an infusion of propofol.
Subsequently, subjects were warmed with one of two randomly assigned methods: 1) forced–air with a full-body cover (Bair Hugger, Augustine Medical, Inc.) set on “high” (≈43°C), or 2) the circulating-water garment which was positioned beneath the volunteers and then wrapped around the anterior surface so it covered the entire torso, legs, and upper arms. The circulating-water garment was set to 37°C and servo-controlled to esophageal temperature. The alternative treatment was used on the second study day in each volunteer. The forced-air warmer covered 64% of the total surface area of the body; the circulating-water garment covered 77.5%.
The start of forced-air or circulating-water warming was designated as elapsed time zero. Rewarming continued for 2.5 hours, until core temperature reached 37°C, or until copious sweating was observed. At the end of the rewarming period, muscle relaxation was antagonized by administration of glycopyrrolate 0.5 mg and neostigmine 5 mg and the trachea extubated.
Measurements
End-tidal desflurane and carbon dioxide concentrations were monitored using a Capnomac Ultima (Datex Medical Instruments, Tewksbury, MA). Blood pressure, arterial saturation, and heart rate were measured using monitors incorporated into an Ohmeda Modulus CD anesthesia machine (Ohmeda, Inc., Salt Lake City, UT). Arterio-venous shunt vasoconstriction was evaluated with forearm minus fingertip and calf minus toe skin-temperature gradients.13 Gradients exceeding 0°C were considered evidence of vasoconstriction since that value is associated with onset of the core-temperature plateau during general anesthesia.14 Core temperature was measured via a thermocouple in the distal esophagus (Tyco-Mallinckrodt Anesthesiology Products, Inc., St. Louis, MO).
Energy expenditure, derived from oxygen consumption and carbon dioxide production, was measured using a metabolic monitor (Vmax™, Sensor Medics Corp., Yorba Linda, CA). The system was calibrated daily using a known mixture of gases. Measurements were averaged over 1-minute intervals and recorded every five minutes. Area-weighted heat flux and temperatures from 15 skin-surface sites were measured using thermal flux transducers (Concept Engineering, Old Saybrook, CT).15
Arm and leg tissue temperatures were determined as previously described.16 Briefly, the length of the thigh (groin to mid-patella) and lower leg (mid-patella to ankle) were measured in cm. Circumference was measured at the mid upper thigh, mid lower thigh, mid upper calf, and mid lower calf. At each circumference, right leg muscle temperatures were recorded using 8-, 18-, and 38-mm-long, 21-g, needle thermocouples (Tyco-Mallinckrodt Anesthesiology Products, Inc.) inserted perpendicular to the skin surface. Skin-surface temperatures were recorded immediately adjacent to each set of needles and directly posterior to each set. Subcutaneous temperature was measured on the ball of the foot and palm using deep-tissue thermometers (Terumo Medical Corp., Tokyo, Japan).17,18 These devices estimate tissue temperature ≈1 cm below the skin surface.
The lengths of the right arm (axilla to elbow) and forearm (elbow to wrist) were measured in cm. The circumference was measured at the mid-point of each segment. As in the right leg, 8-, 18-, and 38-mm-long needle thermocouples were inserted into each segment. Skin-surface temperatures were recorded immediately adjacent to each set of needles. Core, skin-surface, and muscle temperatures were recorded from thermocouples connected to three calibrated Iso-Thermex 16-channel electronic thermometers (Columbus Instruments International, Corp., Columbus, OH, USA). Temperatures and thermal fluxes were measured at one-second intervals, then averaged and recorded every five minutes.
Data Analysis
Oxygen consumption (ml/min) was converted to equivalent metabolic heat production (watts) assuming the caloric value of oxygen to be 4.82 kcal/L (respiratory quotient = 0.82) and using a conversion of 1 kcal/h = 1.16 W. We chose a standard value for the respiratory quotient because the caloric value of oxygen varies only slightly over the full range of respiratory quotients; the use of a standard value thus introduced minimal error in the calculation of metabolic heat production.
As in previous studies,19 measured cutaneous heat loss was reduced 3% to compensate for the skin covered by the volunteers' shorts. We augmented cutaneous loss by 10 kcal/hour (≈10% of the basal metabolic rate) to account for respiratory loss.20 And finally, heat loss on the forced-air day was augmented by 10% to account for insensible transcutaneous evaporative loss. We defined flux as positive when heat traversed skin to the environment. Overall, heat balance was calculated as the difference between heat production and adjusted heat loss. Posterior heat flux was calculated as the area-weighted sum of adjusted flux through the back, posterior thigh, and posterior calf.
The leg was divided into five segments: upper thigh, lower thigh, upper calf, lower calf, and foot. Each thigh and calf segment was further divided into an anterior and posterior section, with one third of the estimated mass considered posterior.
Anterior segment tissue temperatures, as a function of radial distance from the center of the leg segment, were calculated using skin-surface and muscle temperatures using fourth-order regressions. Temperature at the center of the thigh was set to core temperature. In contrast, temperature at the center of the lower leg segments was estimated from the regression equation with no similar assumption. Anterior limb heat content was estimated from these temperatures, as previously described,11 using the following formula:
| (1) |
where Q(0→r) (cal) is heat content of the leg segment from the center to radius r, L (cm) is the length of the leg segment (i.e., groin to mid-thigh, mid-calf to ankle), ρ (g/cm3) is tissue density, s (cal·°C−1·g−1) is the specific heat of leg tissues, a0 (°C) is the temperature at the center of the leg segment, and a2 (°C/cm2) and a4 (°C/cm4) are the regression constants. The specific heat of muscle was taken as 0.89 cal·°C−1·g−1 and density as 1.06 g/cm3.21
Rather than assume full radial symmetry, we assumed only that radial temperature distribution in the posterior leg segments would also be parabolic. Accordingly, we calculated the regression constant a2 in the posterior leg segments from a0 determined from the adjacent anterior segment and the posterior segment skin temperature. Posterior segment tissue heat contents were then determined from Eq. 1. Average segment tissue temperatures were determined by Eq. 2 or its forth-order equivalent, as appropriate.
| (2) |
We have previously described the derivation of these equations, and their limitations.16
Hand and foot volumes were determined in each volunteer by water displacement. “Deep temperature,” measured on the ball of the foot, was assumed to represent the entire foot. Foot heat content thus was calculated by multiplying foot temperature by the mass of the foot and the specific heat of muscle. Average temperatures of the thigh and lower leg (calf and foot) were calculated by weighting values from each of the nine segments in proportion to their estimated masses. The right and left legs were treated comparably throughout this study, so we assumed that average tissue temperatures in the two limbs were similar.
Arm tissue temperature and heat content were calculated from parabolic tissue temperature regressions and the above equations. In the arms, we assumed full radial symmetry and thus did not separately calculate posterior segment values. Palm “deep temperature” was assumed to represent that of the entire hand. Hand heat content thus was calculated by multiplying deep palm temperature by the mass of the hand and the specific heat of muscle. As in the leg, average temperatures of the arm and forearm (forearm and hand) were calculated by weighting values from each of the three segments in proportion to their estimated masses.
Changes in trunk and head heat content were modeled simply by multiplying the weight of the trunk and head by the change in core temperature and the average specific heat of human tissues. Trunk and head weight was estimated by subtracting the calculated weight of the extremities (from the radial integration) from the total weight of each subject.
Values during the control periods were first averaged within each volunteer and then averaged among the volunteers. Potential confounding factors were compared with paired t-tests and are expressed as means ± standard deviations. The rate at which core temperature increased was evaluated by regression from 60 to 120 elapsed minutes (the linear portion of the curve). Results comparing temperature and heat content between the two treatments are expressed as means with 95% confidence intervals and displayed in the figures. Differences were considered statistically significant when P < 0.05.
Results
The volunteers were 26 ± 4 years old, weighed 70 ± 12 kg, and were 175 ± 10 cm tall. Estimated mass of the thighs and lower legs (including feet) were 20 ± 4 kg and 8 ± 1 kg, respectively. Consequently, the legs represented 40% of our volunteers' total mass. Similarly, estimated masses of the arms and forearms (including hands) were 4 ± 1 kg and 3 ± 1 kg, respectively. Consequently, the arms represented ≈10% of the volunteers' total mass. The peripheral thermal compartment (extremities) thus represented 50% of our volunteers' mass.
Anesthetic management was similar on each study day. Ambient temperatures were also similar. It took 80 ± 31 minutes for the subjects to cool to 34°C. Conditions during the control periods preceding each warming treatment were comparable (Table 1). The respiratory quotient was 0.8 ± 0.2 and did not change significantly during the study period. Mean arterial pressure differed significantly before each treatment, but only by 4 mmHg.
Table 1.
Temperatures and Hemodynamic Responses During the Control Period.
| Circulating-Water | Forced-Air | P | |
|---|---|---|---|
| Ambient Temperature (°C) | 20.4 ± 1.3 | 21.0 ± 1.9 | 0.39 |
| Mean Arterial Pressure (mmHg) | 94 ± 12 | 90 ± 10 | 0.05 |
| Heart Rate (beats per min) | 54 ± 7 | 54 ± 8 | 0.99 |
| Forearm – Calf Gradient (°C) | 0 ± 1.2 | 1 ± 1.5 | 0.11 |
| Forearm-Fingertip Gradient (°C) | 3.6 ± 0.6 | 3.4 ± 0.9 | 0.43 |
| Mean Skin Temperature (°C) | 30.7 ± 0.9 | 31.2 ± 0.7 | 0.16 |
Data presented as mean ± SD.
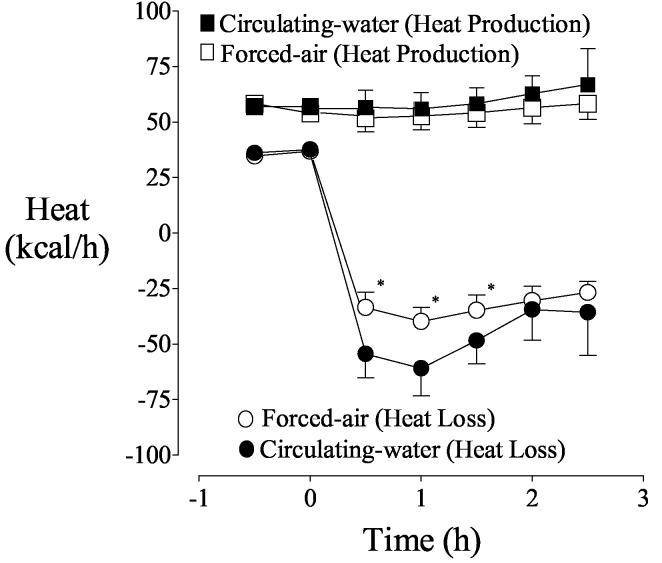
Initial heat production was near 60 kcal/h with each treatment and remained essentially unchanged for the duration of the study. Heat loss during the control period was also similar with each treatment. Heat gain from anterior portions of the skin surface was similar with each warming system. For example, anterior heat gain after 0.5 hours of warming was 26 ± 9 kcal/h with forced air and 26 ± 8 kcal/h with circulating water.
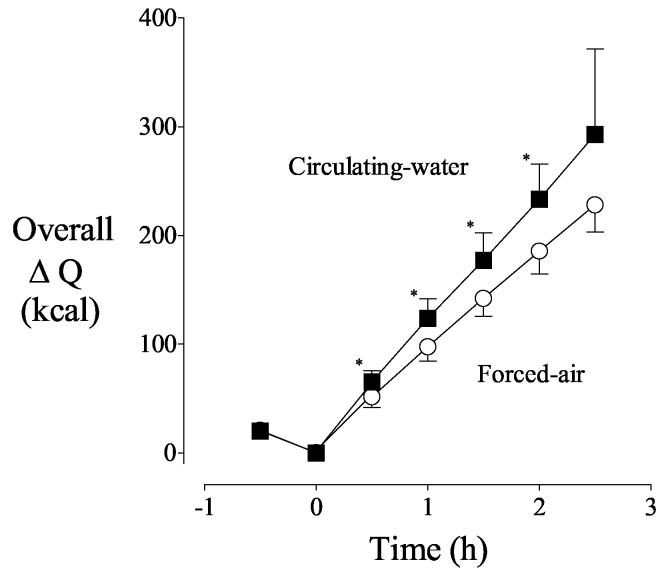
Forced-air warmers heat only the anterior surface of patients. As might thus be expected, there was little heat exchange through posterior skin into the foam insulation covering the operating-room table on the forced-air day. Circulating water, in contrast, transferred 21 ± 9 kcal/h through the posterior skin surface after a half hour of warming. However, it is important to recognize that initial loss from the posterior surface was tiny whereas substantial heat was lost anteriorly. The anterior skin surface was responsible for 94% of the increase in cutaneous heat transfer in the first half hour of warming with forced air whereas the anterior surface was responsible for only 71% of the increase with circulating water. Virtually the entire difference between the two warming systems was therefore explained by posterior heat transfer. Because the heat transfer rate was greater with circulating water (Fig. 1), overall body heat content increased more quickly with circulating water (Fig. 2).
Fig. 1.
Heat production and cutaneous heat loss before and during warming. Circulating-water or forced-air warming began at elapsed time zero. Results are presented as means with 95% confidence intervals. Heat production was similar throughout the study. Asterisks (*) indicate times when heat loss differed significantly between the two treatment days, P < 0.05.
Fig. 2.
Systemic heat balance, as determined by the difference between heat production and heat loss before and during warming. Circulating-water or forced-air warming began at elapsed time zero. Results are presented as means with 95% confidence intervals. Asterisks (*) indicate the times when the change in overall heat content was significantly greater with the circulating-water garment, P < 0.05.
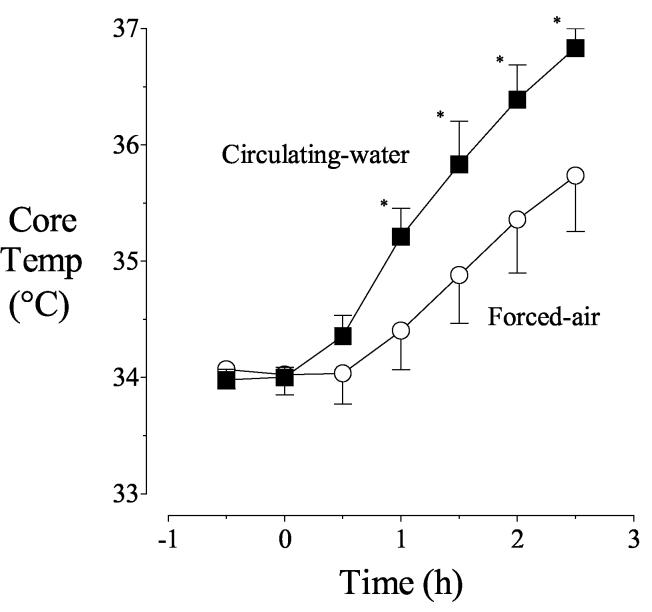
Core temperatures were comparable and nearly constant near 34°C before warming was started. Core temperature remained unchanged for the first half hour of forced-air warming and had increased only by 0.4 ± 1.5°C after the first hour. In contrast, core temperature began to increase immediately with the circulating-water garment and had increased by 1.2 ± 0.3°C after the first hour (P < 0.001). However, core temperature increased similarly between 60 and 120 elapsed minutes: 1.0± 0.5°C/h with forced air and 1.2 ± 0.1°C/h with circulating water (P =0.03). Consequently, after 2.5 hours of warming, core temperatures with the two treatments differed by 1.1 ± 0.7°C (Fig. 3). Core temperature in the forced-air warming group did not reach 37°C even after 2.5 hours of warming.
Fig. 3.
Core temperatures before and after warming. Circulating-water or forced-air warming began at elapsed time zero. Results are presented as means with 95% confidence intervals. Asterisks (*) indicate times when core temperatures differed significantly on the treatment days, P < 0.05.
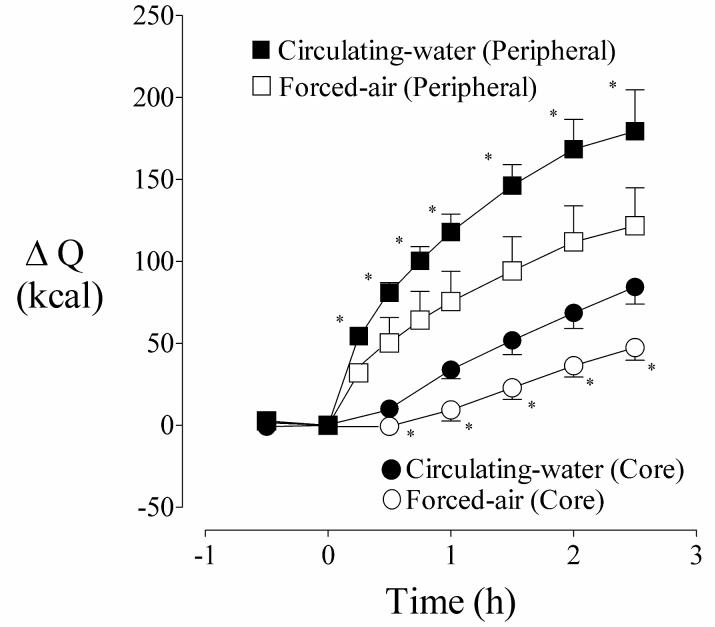
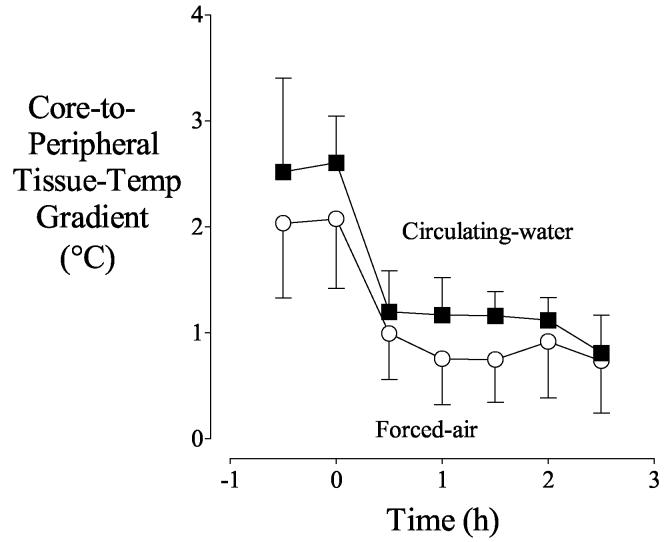
During the first hour of warming, peripheral-tissue heat content increased faster than core heat content (Fig. 4) with each device. The core-to-peripheral tissue temperature gradient therefore decreased markedly during this first hour. However, the increases in peripheral and core heat content were subsequently similar. Consequently, the core-to-peripheral tissue temperature gradient also was constant (Fig. 5).
Fig. 4.
Cumulative changes in measured peripheral (extremities) and core (trunk) heat content before and during warming. Circulating-water or forced-air warming began at elapsed time zero. Results are presented as means with 95% confidence intervals. Asterisks (*) denote the times when changes in peripheral and core heat contents differed significantly between the treatment days, P < 0.05
Fig. 5.
Core-to-peripheral tissue temperature gradient before and during warming. Average temperature of extremity tissues was considered peripheral temperature. Circulating-water or forced-air warming began at elapsed time zero. Results are presented as means with 95% confidence intervals. Although the gradient was slightly greater with circulating-water even before active warming, the shape of the curves was similar.
Discussion
Differences in perioperative patient warming systems result largely from what tissues are in contact with what heating element and the available surface area. Heat transfer also depends on physical characteristics of the heater-skin interface. For example, the surface area of the lung is enormous, but airway heaters and humidifiers transfer trivial amounts of heat because the thermal capacity of air is small.
With any cutaneous warming system, heat transfer into the thermal core depends on skin temperature, tissue insulation, and circulatory convection of heat within the body. Device efficacy thus depends on which surface area is heated because the core is relatively isolated from distal skin surfaces. But most importantly, cutaneous heat transfer depends on skin temperature. Nearly all commercially available patient-warming systems are electrically powered; there is, therefore, no intrinsic physical limit to the calories that can be provided. Instead, the limitation is always the skin temperature that can be tolerated without undue risk of burns.
Despite the high heat capacity and thermal conductivity of water, the efficacy of conventional circulating-water mattresses is modest. Poor efficacy results because 1) the posterior surface is a relatively small fraction of the body surface area, 2) this area is poorly perfused because the weight of the body compresses cutaneous capillaries, and, 3) most heat is lost via radiation and convection from the anterior surfaces rather than conduction into the operating-table mattress. As might thus be expected, the circulating-water garment transferred only 21 kcal/h across the posterior skin surface. This is more than reported previously with a conventional circulating-water mattress,22 possibly because of a better interface material. However, it is roughly the same change in cutaneous heat transfer that is provided by a single cotton blanket in a normothermic subject.15
Anterior surface heat transfer was comparable with each warming system, and the change in anterior surface heat gain from 0 to 0.5 elapsed hours averaged ≈65 kcal/h with each treatment. Heat transfer per anterior unit area was thus similar with each system. A corollary of this observation is that virtually the entire difference between the two tested warming systems resulted from heat transfer into posterior surfaces, that is from the portion of the circulating-water garment that acts as mattress. Core temperature increased 0.4°C/h faster with circulating water than forced air, a result that is consistent with Janicki et al.8 Although not tested in this study, our results suggest that heat transfer and core rewarming with the circulating-water garment would be similar to that provided by combining a forced-air cover and a conventional circulating-water mattress.
The core and peripheral thermal compartments were of similar size (e.g., weight). However, active warming increased peripheral tissue heat content roughly three times as much as the core over the course of the study. The differences were even more pronounced during the initial warming phase. For example, peripheral heat content after one hour of circulating water increased 114 kcal whereas core content increased only 34 kcal. The analogous values for forced air were 71 and 9 kcal. Peripheral compartment heat content thus increased 60-80 kcal more than the core compartment with each device. These data indicate that tissue insulation restricted rapid flow of heat from the periphery to the core. In other words, applied heat was constrained by the insulating properties of peripheral tissues, thus significantly limiting the rate at which core temperature increased.
That peripheral tissues insulated the core and slowed heat transfer in our volunteers is consistent with observations of Plattner et al. who found that peripheral tissues isolate the core from heat applied to the skin surface in the post-anesthetic period.12 Similarly, Szmuk et al. found that core rewarming was slowed by postoperative vasoconstriction.23 In contrast, peripheral-to-core heat transfer is unimpeded during anesthesia,19 whether subjects are vasodilated or vasoconstricted.24 The critical distinction amongst these studies is that volunteers were fully anesthetized in the later protocols whereas they were unanesthetized in the former ones. Although our volunteers remained intubated, they were very lightly anesthetized and fully vasoconstricted. It is thus unlikely that they were given sufficient anesthesia to cause direct arteriolar vasodilation that seems to be critical for rapid peripheral-to-core heat transfer.
Although core temperatures were virtually identical at onset of warming, peripheral tissue temperature was slightly cooler on the circulating-water day. This lower initial skin temperature and greater initial core-to-peripheral tissue-temperature gradient increases the apparent efficacy of circulating water. However, the tissue temperature difference was only a few tenths of a °C and thus unlikely to have substantially altered the results.
Traditional circulating-water mattresses are associated with “pressure-heat necrosis” (i.e., burn) that results when tissue compressed by the weight of the patient is simultaneously warmed.25-28 Gali et al.29 recently reported the case of a 67-year-old woman who developed burns on her back after 6.5 hours of surgery while being warmed with the same circulating-water garment we used. Thus, when using this system, clinicians should consider any risk factors such as age, length of surgery, and nutritional status, which may predispose a patient to skin injury.
In summary, the circulating-water garment transferred more heat than forced air, especially during the first hour of warming, with the difference resulting largely from posterior heating. Excessive heating of peripheral thermal compartment indicates that peripheral tissues insulated the core, thus slowing heat transfer.
Footnotes
Received from the Department of Anesthesiology, Washington University — St. Louis, Missouri; the Outcomes Research™ Institute and Department of Anesthesiology and Pharmacology, University of Louisville, Louisville, KY.
Supported by MTRE, Inc. (Or Akiva, Israel), National Institutes of Health Grants GM 58273 and GM 061655 (Bethesda, MD), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY). We appreciate the statistical assistance of Gilbert Haugh, M.A., Outcomes Research™ Institute, University of Louisville.
Summary Statement: Anterior heat transfer with forced-air and circulating-water systems was similar. However, circulating-water provided additional posterior warming which increased total heat transfer. Core warming was thus faster with circulating water.
References
- 1.Sessler DI. Perioperative hypothermia. N Engl J Med. 1997;336:1730–1737. doi: 10.1056/NEJM199706123362407. [DOI] [PubMed] [Google Scholar]
- 2.Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, Beattie C. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: A randomized clinical trial. JAMA. 1997;277:1127–1134. [PubMed] [Google Scholar]
- 3.Kurz A, Sessler DI, Lenhardt RA. Study of wound infections and temperature group: Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 4.Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild intraoperative hypothermia increases blood loss and allogeneic transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–292. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- 5.Giesbrecht GG, Ducharme MB, McGuire JP. Comparison of forced-air patient warming systems for perioperative use. Anesthesiology. 1994;80:671–679. doi: 10.1097/00000542-199403000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Kurz A, Kurz M, Poeschl G, Faryniak B, Redl G, Hackl W. Forced-air warming maintains intraoperative normothermia better than circulating-water mattresses. Anesth Analg. 1993;77:89–95. doi: 10.1213/00000539-199307000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Borms SF, Engelen SLE, Himpe DGA, Suy MR, Theunissen WJH. Bair Hugger forced-air warming maintains normothermia more effectively than Thermo-Lite insulation. J Clin Anesth. 1994;6:303–307. doi: 10.1016/0952-8180(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 8.Janicki PK, Higgins MS, Janssen J, Johnson RF, Beattie C. Comparison of two different temperature maintenance strategies during open abdominal surgery: upper body forced-air warming versus whole body water garment. Anesthesiology. 2001;95:868–74. doi: 10.1097/00000542-200110000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Nesher N, Wolf T, Kushnir I, David M, Bolotin G, Sharony R, Pizov R, Uretzky G. Novel thermoregulation system for enhancing cardiac function and hemodynamics during coronary artery bypass graft surgery. Ann Thorac Surg. 2001;72:S1069–76. doi: 10.1016/s0003-4975(01)02943-5. [DOI] [PubMed] [Google Scholar]
- 10.Nesher N, Wolf T, Uretzky G, Oppenheim-Eden A, Yussim E, Kushnir I, Shoshany G, Rosenberg B, Berant M. A novel thermoregulatory system maintains perioperative normothermia in children undergoing elective surgery. Paediatr Anaesth. 2001;11:555–60. doi: 10.1046/j.1460-9592.2001.00713.x. [DOI] [PubMed] [Google Scholar]
- 11.Matsukawa T, Sessler DI, Sessler AM, Schroeder M, Ozaki M, Kurz A, Cheng C. Heat flow and distribution during induction of general anesthesia. Anesthesiology. 1995;82:662–673. doi: 10.1097/00000542-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Plattner O, Ikeda T, Sessler DI, Christensen R, Turakhia M. Postanesthetic vasoconstriction slows postanesthetic peripheral-to-core transfer of cutaneous heat, thereby isolating the core thermal compartment. Anesth Analg. 1997;85:899–906. doi: 10.1097/00000539-199710000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Rubinstein EH, Sessler DI. Skin-surface temperature gradients correlate with fingertip blood flow in humans. Anesthesiology. 1990;73:541–545. [PubMed] [Google Scholar]
- 14.Kurz A, Sessler DI, Christensen R, Dechert M. Heat balance and distribution during the core-temperature plateau in anesthetized humans. Anesthesiology. 1995;83:491–499. doi: 10.1097/00000542-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Sessler DI, Schroeder M. Heat loss in humans covered with cotton hospital blankets. Anesth Analg. 1993;77:73–77. doi: 10.1213/00000539-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Belani K, Sessler DI, Sessler AM, Schroeder M, McGuire J, Washington D, Moayeri A. Leg heat content continues to decrease during the core temperature plateau in humans. Anesthesiology. 1993;78:856–863. doi: 10.1097/00000542-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Togawa T, Nemoto T, Yamazaki T, Kobayashi T. A modified internal temperature measurement device. Med Biol Eng. 1976;14:361–364. doi: 10.1007/BF02478138. [DOI] [PubMed] [Google Scholar]
- 18.Fox RH, Solman AJ. A new technique for monitoring the deep body temperature in man from the intact skin surface. J Physiol (US) 1970;212:8–10. [PubMed] [Google Scholar]
- 19.Plattner O, Xiong J, Sessler DI, Christensen R, Turakhia M, Dechert M, Clough D. Rapid core-to-peripheral tissue heat transfer during cutaneous cooling. Anesth Analg. 1996;82:925–930. doi: 10.1097/00000539-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Bickler P, Sessler DI. Efficiency of airway heat and moisture exchangers in anesthetized humans. Anesth Analg. 1990;71:415–418. [PubMed] [Google Scholar]
- 21.Burton AC. Human calorimetry: The average temperature of the tissues of the body. J Nutr. 1935;9:261–280. [Google Scholar]
- 22.Hynson J, Sessler DI. Intraoperative warming therapies: A comparison of three devices. J Clin Anesth. 1992;4:194–199. doi: 10.1016/0952-8180(92)90064-8. [DOI] [PubMed] [Google Scholar]
- 23.Szmuk P, Ezri T, Sessler DI, Stein A, Geva D. Spinal anesthesia only minimally increases the efficacy of postoperative forced-air rewarming. Anesthesiology. 1997;87:1050–1054. doi: 10.1097/00000542-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Clough D, Kurz A, Sessler DI, Christensen R, Xiong J. Thermoregulatory vasoconstriction does not impede core warming during cutaneous heating. Anesthesiology. 1996;85:281–288. doi: 10.1097/00000542-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Gendron F. “Burns” occurring during lengthy surgical procedures. J Clin Engineer. 1980;5:20–26. doi: 10.1097/00004669-198001000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Gendron FG. Unexplained Patient Burns: Investigating Iatrogenic Injuries. Quest Publishing Co., Inc.; Brea, CA: 1988. [Google Scholar]
- 27.Crino MH, Nagel EL. Thermal burns caused by warming blankets in the operating room. Anesthesiology. 1968;29:149–151. doi: 10.1097/00000542-196801000-00038. [DOI] [PubMed] [Google Scholar]
- 28.Scott SM, Oteen NC. Thermal blanket injury in the operating room. Arch Surg. 1967;94:181. doi: 10.1001/archsurg.1967.01330080019006. [DOI] [PubMed] [Google Scholar]
- 29.Gali B, Findlay JY, Plevak DJ. Skin injury with the use of a water warming device. Anesthesiology. 2003;98:1509–10. doi: 10.1097/00000542-200306000-00032. [DOI] [PubMed] [Google Scholar]