Abstract
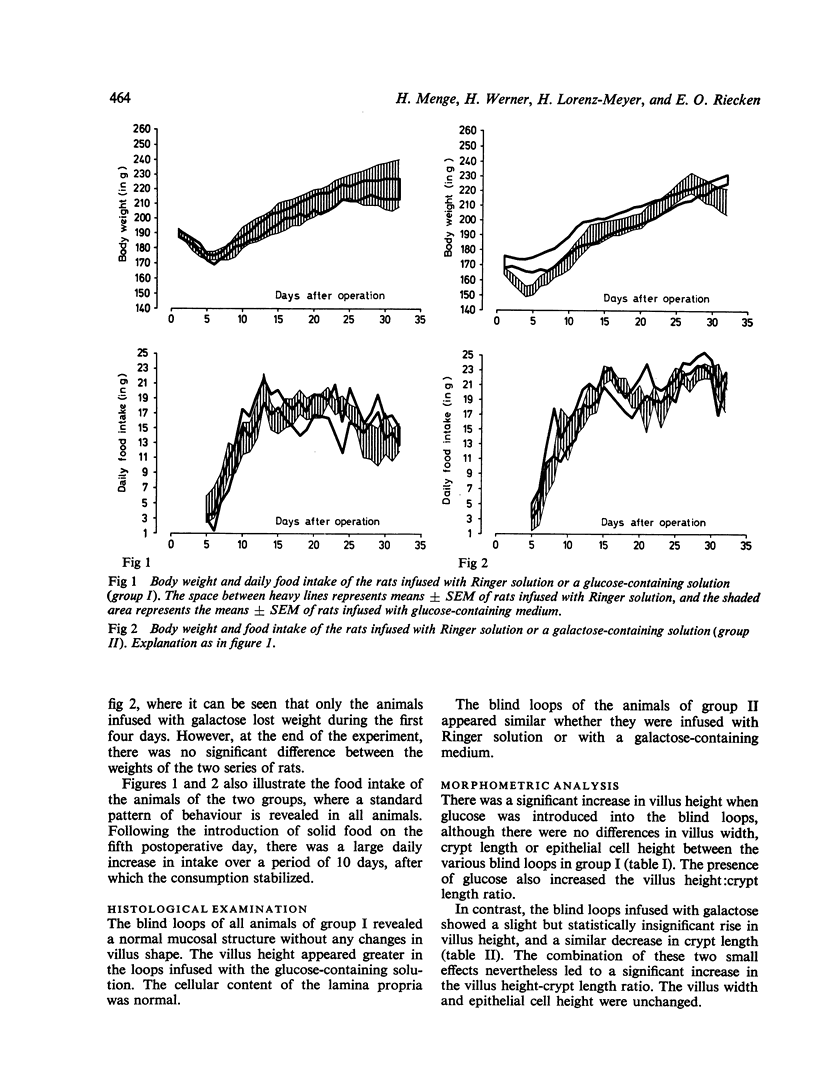
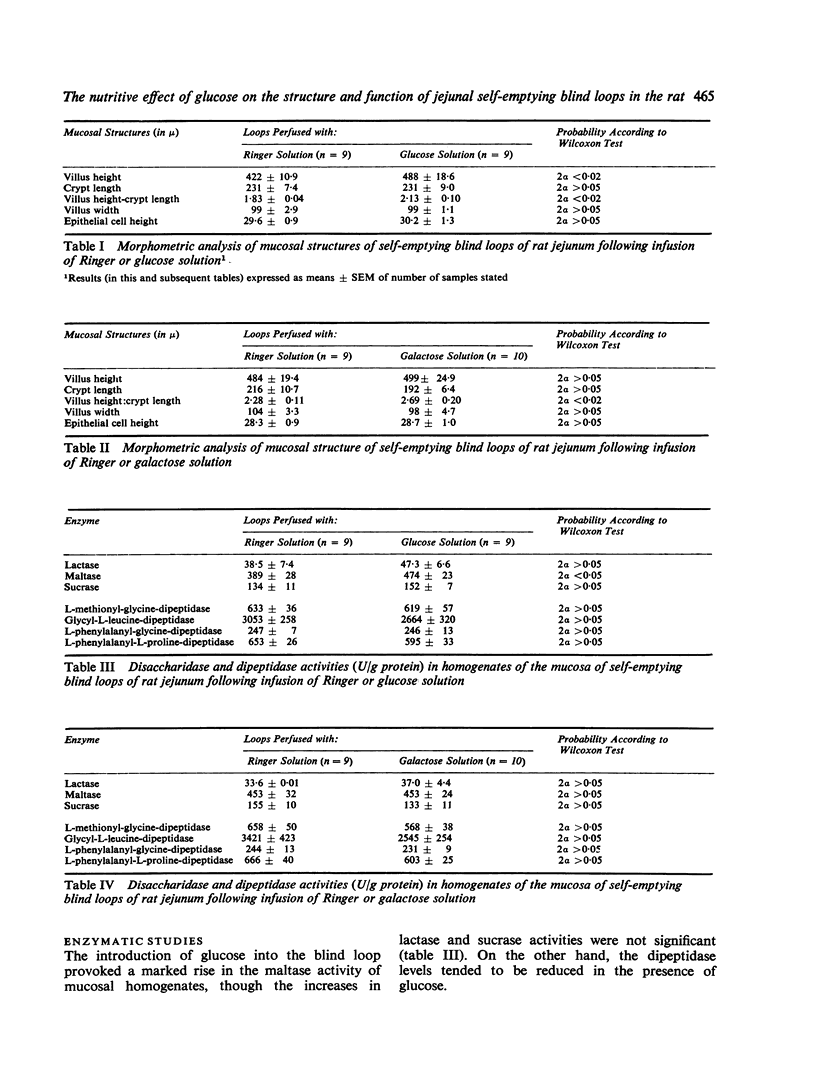
In an attempt to obtain further information on the influence of the intestinal contents on the development of mucosal structure and function, self-emptying blind loops of rat jejunum were constructed, and the oral end was exteriorized with a stoma to permit infusion of different solutions into the loop. Ringer solution or media containing glucose or galactose was instilled into the loops three times daily for 27 days before functional and structural examination of the loops. The body weight and food intake of the animals did not differ significantly from one group to another. Treatment with glucose, but not with galactose or Ringer solution alone, induced a significant increase in the villus height. Disaccharidase but not dipeptidase activity was concomitantly increased. Infusion of glucose or galactose both led to an increase in the transport capacity of the loop, as determined by glucose absorption in vivo. These results indicate that glucose has a nutritive effect, probably due to its intracellular metabolism, which is responsible for the structural alterations observed. On the other hand, the increase in transport capacity may be attributed to substrate-induced stimulation of the specific monosaccharide transport system in the epithelial cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auricchio S., Pierro M., Orsatti M. Assay of peptidase activities of intestinal brush border membrane with L-amino acid oxidase. Anal Biochem. 1971 Jan;39(1):15–23. doi: 10.1016/0003-2697(71)90456-8. [DOI] [PubMed] [Google Scholar]
- BOOTH C. C., EVANS K. T., MENZIES T., STREET D. F. Intestinal hypertrophy following partial resection of the small bowel in the rat. Br J Surg. 1959 Jan;46(198):403–410. doi: 10.1002/bjs.18004619821. [DOI] [PubMed] [Google Scholar]
- BORGSTROM B., DAHLQVIST A., LUNDH G., SJOVALL J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957 Oct;36(10):1521–1536. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R. J., Eggenton J., Smyth D. H. Sodium pumps in the rat small intestine in relation to hexose transfer and metabolism. J Physiol. 1969 Oct;204(2):299–310. doi: 10.1113/jphysiol.1969.sp008914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Galjaard H., Bootsma D. The regulation of cell proliferation and differentiation in intestinal epithelium. II. A quantitative histochemical and autoradiographic study after low doses of x-irradiation. Exp Cell Res. 1969 Nov;58(1):79–92. doi: 10.1016/0014-4827(69)90118-9. [DOI] [PubMed] [Google Scholar]
- Gleeson M. H., Cullen J., Dowling R. H. Intestinal structure and function after small bowel by-pass in the rat. Clin Sci. 1972 Dec;43(6):731–742. doi: 10.1042/cs0430731. [DOI] [PubMed] [Google Scholar]
- HYVARINEN A., NIKKILA E. A. Specific determination of blood glucose with o-toluidine. Clin Chim Acta. 1962 Jan;7:140–143. doi: 10.1016/0009-8981(62)90133-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Menge H., Bloch R., Lorenz-Meyer H., Riecken E. O. Morphologie und Funktion isoperistaltischer, ausgeschalteter Jejunalschlingen nach Rückverlegung in die Dünndarmpassage. Res Exp Med (Berl) 1973 Aug 22;161(2):133–140. doi: 10.1007/BF01855105. [DOI] [PubMed] [Google Scholar]
- Menge H., Bloch R., Schaumlöffel E., Riecken E. O. Transportstudien, morphologische, morphometrische und histochemische Untersuchungen zum Verhalten der Dünndarmschleimhaut im operativ ausgeschalteten Jejunalabschnitt der Ratte. Z Gesamte Exp Med. 1970;153(1):74–90. [PubMed] [Google Scholar]
- Rosensweig N. S., Herman R. H. Control of jejunal sucrase and maltase activity by dietary sucrose or fructose in man. A model for the study of enzyme regulation in man. J Clin Invest. 1968 Oct;47(10):2253–2262. doi: 10.1172/JCI105910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharrer E. Rückbildung des aktiven intestinalen Glucosetransports beim Wiederkäuer. Z Tierphysiol Tierernahr Futtermittelkd. 1974 Feb;32(6):320–328. [PubMed] [Google Scholar]
- Scharrer E. Untersuchungen zur Resorption von D-Glucose und L-Leucin am isolierten Jejunum von Lämmern. Zentralbl Veterinarmed A. 1973 Oct;20(8):683–691. [PubMed] [Google Scholar]


