Abstract
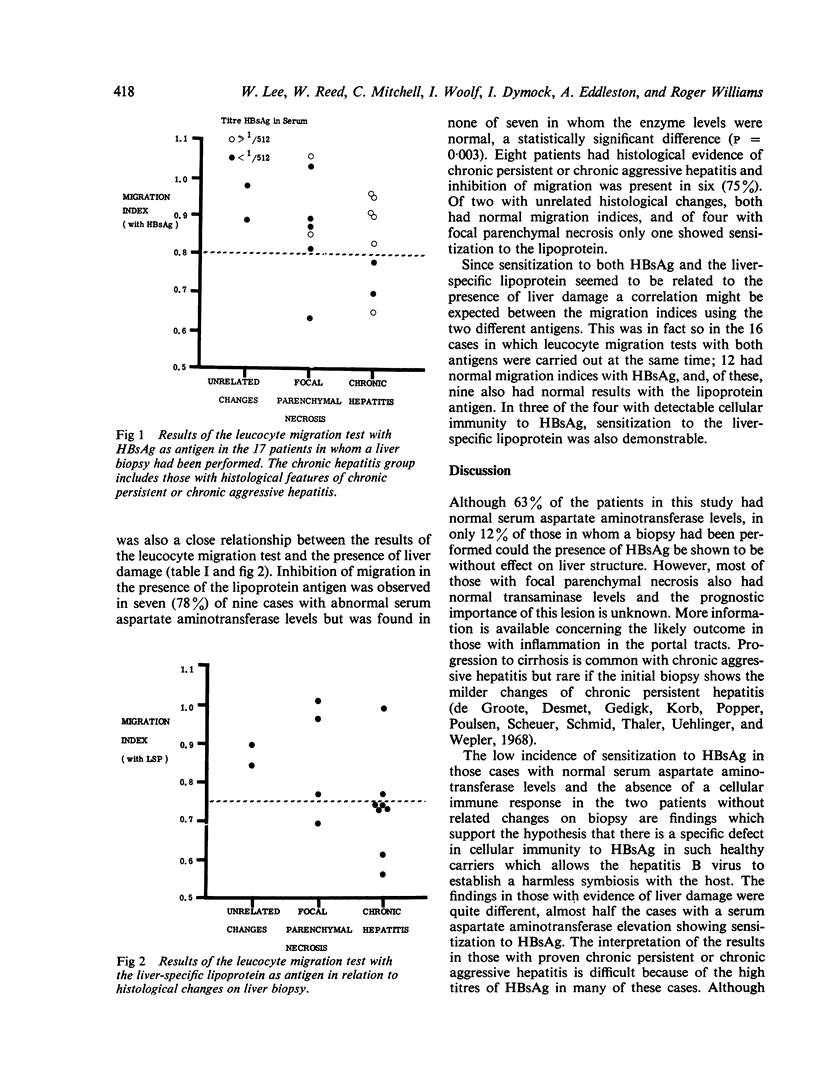
Cellular immunity to the hepatitis B surface antigen (HBsAg) and a liver-specific lipoprotein was studied, using the leucocyte migration test, in 38 asymptomatic blood donors found to have HBsAg in the serum. Sensitization to HBsAg was found in 26% and was related to the presence of liver damage, being detected in 47% of those with elevated serum aspartate aminotransferase but in only 13% with normal enzyme levels. The frequency of sensitization to this antigen in those with chronic persistent or chronic aggressive hepatitis on biopsy was also higher than in those with unrelated or minimal changes. The findings using the liver-specific lipoprotein as antigen were similar and there was a correlation between the results obtained with this and the hapatitis B surface antigen. This study supports the hypothesis that a T-lymphocyte response to hepatitis B virus antigen can initiate an autoimmune reaction to antigens such as liver-specific lipoprotein on the hepatocyte surface, and that this reaction may be of importance in the production of chronic liver damage. In the absence of the T-cell response, the autoimmune reaction cannot occur and the virus is able to establish a harmless symbiotic union with the host.
Full text
PDF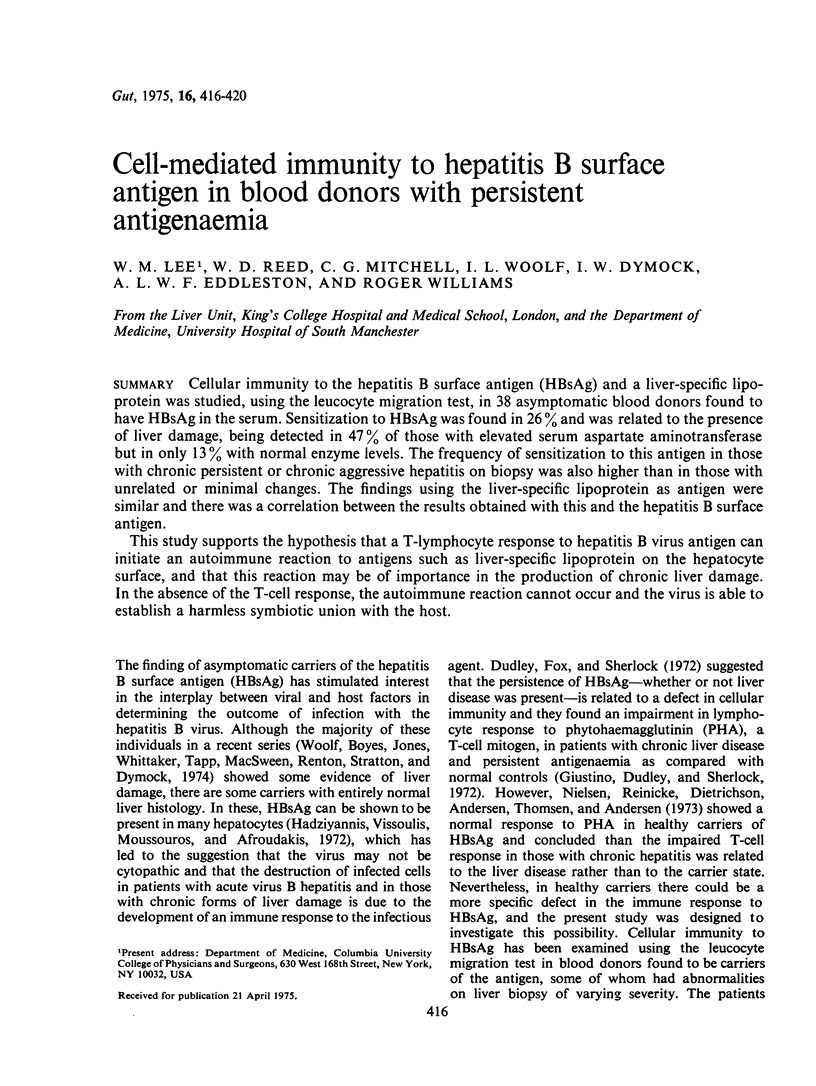



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Büschenfelde K. H., Kössling F. K., Miescher P. A. Experimental chronic active hepatitis in rabbits following immunization with human liver proteins. Clin Exp Immunol. 1972 May;11(1):99–108. [PMC free article] [PubMed] [Google Scholar]
- De Groote J., Desmet V. J., Gedigk P., Korb G., Popper H., Poulsen H., Scheuer P. J., Schmid M., Thaler H., Uehlinger E. A classification of chronic hepatitis. Lancet. 1968 Sep 14;2(7568):626–628. doi: 10.1016/s0140-6736(68)90710-1. [DOI] [PubMed] [Google Scholar]
- Dudley F. J., Fox R. A., Sherlock S. Cellular immunity and hepatitis-associated, Australia antigen liver disease. Lancet. 1972 Apr 1;1(7753):723–726. doi: 10.1016/s0140-6736(72)90234-6. [DOI] [PubMed] [Google Scholar]
- Eddleston A. L., McFarlane I. G., Mitchell C. G., Reed W. D., Williams R. Cell-mediated immune response in primary biliary cirrhosis to a protein fraction from human bile. Br Med J. 1973 Nov 3;4(5887):274–276. doi: 10.1136/bmj.4.5887.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston A. L., Williams R. Inadequate antibody response to hBAg or suppressor T-cell defect in development of active chronic hepatitis. Lancet. 1974 Dec 28;2(7896):1543–1545. doi: 10.1016/s0140-6736(74)90287-6. [DOI] [PubMed] [Google Scholar]
- Giustino V., Dudley F. J., Sherlock S. Thymus-dependent lymphocyte function in patients with hepatitis-associated antigen. Lancet. 1972 Oct 21;2(7782):850–853. doi: 10.1016/s0140-6736(72)92212-x. [DOI] [PubMed] [Google Scholar]
- Hadziyannis S., Moussouros A., Vissoulis C., Afroudakis A. Cytoplasmic localisation of Australia antigen in the liver. Lancet. 1972 May 6;1(7758):976–979. doi: 10.1016/s0140-6736(72)91153-1. [DOI] [PubMed] [Google Scholar]
- Laiwah A. A. Lymphocyte transformation by Australia antigen. Lancet. 1971 Aug 28;2(7722):470–471. doi: 10.1016/s0140-6736(71)92633-x. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Reed W. D., Mitchell C. G., Galbraith R. M., Eddleston A. L., Zuckerman A. J., Williams R. Cellular and humoral immunity to hepatitis-B surface antigen in active chronic hepatitis. Br Med J. 1975 Mar 29;1(5960):705–708. doi: 10.1136/bmj.1.5960.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. O., Reinicke V., Dietrichson O., Andersen V., Thomsen M., Andersen E. Immunological studies of Australia antigen carriers with and without liver diseases. Clin Exp Immunol. 1973 Sep;15(1):9–16. [PMC free article] [PubMed] [Google Scholar]
- Reed W. D., Mitchell C. G., Eddleston A. L., Lee W. M., Williams R., Zuckerman A. J. Exposure and immunity to hepatitis-B virus in a liver unit. Lancet. 1974 Apr 6;1(7858):581–583. doi: 10.1016/s0140-6736(74)92646-4. [DOI] [PubMed] [Google Scholar]
- Reed W. D., Stern R. B., Eddleston A. L., Williams R., Zuckerman A. J., Bowes A., Earl P. M. Detection of hepatitis-B antigen by radioimmunoassay in chronic liver disease and hepatocellular carcinoma in Great Britain. Lancet. 1973 Sep 29;2(7831):690–694. doi: 10.1016/s0140-6736(73)92534-8. [DOI] [PubMed] [Google Scholar]
- Thomson A. D., Cochrane M. A., McFarlane I. G., Eddleston A. L., Williams R. Lymphocyte cytotoxicity to isolated hepatocytes in chronic active hepatitis. Nature. 1974 Dec 20;252(5485):721–722. doi: 10.1038/252721a0. [DOI] [PubMed] [Google Scholar]
- Woolf I. L., Boyes B. E., Jones D. M., Whittaker J. S., Tapp E., MacSween R. N., Renton P. H., Stratton F., Dymock I. W. Asymptomatic liver disease in hepatitis B antigen carriers. J Clin Pathol. 1974 May;27(5):348–352. doi: 10.1136/jcp.27.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]


