Abstract
The use of adeno-associated virus type 2 (AAV) vectors has gained attention as a potentially useful alternative to the more commonly used retrovirus and adenovirus vectors for human gene therapy. However, the transduction efficiency of AAV vectors varies greatly in different cells and tissues in vitro and in vivo. We have documented that a cellular protein that binds the immunosuppressant drug FK506, termed the FK506-binding protein (FKBP52), interacts with the single-stranded D sequence within the AAV inverted terminal repeats, inhibits viral second-strand DNA synthesis, and consequently limits high-efficiency transgene expression (K. Qing, J. Hansen, K. A. Weigel-Kelley, M. Tan, S. Zhou, and A. Srivastava, J. Virol., 75: 8968-8976, 2001). FKBP52 can be phosphorylated at both tyrosine and serine/threonine residues, but only the phosphorylated forms of FKBP52 interact with the D sequence. Furthermore, the tyrosine-phosphorylated FKBP52 inhibits AAV second-strand DNA synthesis by greater than 90%, and the serine/threonine-phosphorylated FKBP52 causes ∼40% inhibition, whereas the dephosphorylated FKBP52 has no effect on AAV second-strand DNA synthesis. In the present study, we have identified that the tyrosine-phosphorylated form of FKBP52 is a substrate for the cellular T-cell protein tyrosine phosphatase (TC-PTP). Deliberate overexpression of the murine wild-type (wt) TC-PTP gene, but not that of a cysteine-to-serine (C-S) mutant, caused tyrosine dephosphorylation of FKBP52, leading to efficient viral second-strand DNA synthesis and resulting in a significant increase in AAV-mediated transduction efficiency in HeLa cells in vitro. Both wt and C-S mutant TC-PTP expression cassettes were also used to generate transgenic mice. Primitive hematopoietic stem/progenitor cells from wt TC-PTP-transgenic mice, but not from C-S mutant TC-PTP-transgenic mice, could be successfully transduced by recombinant AAV vectors. These studies corroborate the fact that tyrosine phosphorylation of the cellular FKBP52 protein strongly influences AAV transduction efficiency, which may have important implications in the optimal use of AAV vectors in human gene therapy.
Adeno-associated virus type 2 (AAV) is a nonpathogenic human parvovirus that contains a single-stranded DNA as its genome and requires coinfection with a helper virus, usually adenovirus, for its optimal replication (2, 28). In the absence of coinfection with the helper virus, the wild-type (wt) AAV establishes a latent infection and the viral genome integrates into human chromosomal DNA in a site-specific manner (19, 20, 38). The nonpathogenicity of AAV and the remarkable site specificity of its integration have led to the development of recombinant AAV vectors for gene transfer and gene therapy. Although recombinant AAV genomes appear not to integrate site specifically, AAV vectors have been successfully used to deliver genes to a wide variety of cells and tissues in vitro and in vivo (3, 4, 10, 11, 14-18, 26, 29-33, 39-41, 44-46, 48). AAV vectors have also been used in phase I clinical trials for gene therapy of cystic fibrosis and hemophilia B (10, 16). However, the transduction efficiency of AAV vectors has been reported to vary widely in different cell types. Two independent laboratories have reported that the rate-limiting step in transduction by AAV vectors is viral second-strand DNA synthesis (8, 9). We have previously documented the existence of a host cell protein that we designated the single-stranded D sequence-binding protein (ssD-BP), which interacts specifically with the D sequence within the inverted terminal repeat of the AAV genome, is phosphorylated at tyrosine residues by the cellular epidermal growth factor receptor protein tyrosine kinase EGFR-PTK, and inhibits viral second-strand DNA synthesis leading to inefficient transgene expression (22, 23, 34, 36, 37). We subsequently identified the ssD-BP to be FKBP52, a cellular chaperone protein (36).
In this report, we present evidence to document that the cellular protein that binds the immunosuppressant drug FK506, termed the FK506-binding protein (FKBP52), is dephosphorylated at tyrosine residues by the cellular T-cell protein tyrosine phosphatase (TC-PTP) (21, 47). Stable transfection of a murine TC-PTP expression plasmid catalyzes tyrosine dephosphorylation of FKBP52, leads to efficient viral second-strand DNA synthesis, and results in a significant increase in AAV-mediated transduction efficiency in established human cell lines as well as in primary cells from TC-PTP-transgenic mice. These studies have important implications in the optimal use of AAV vectors in human gene therapy.
Deliberate expression of TC-PTP leads to increased AAV-mediated transgene expression in HeLa cells.
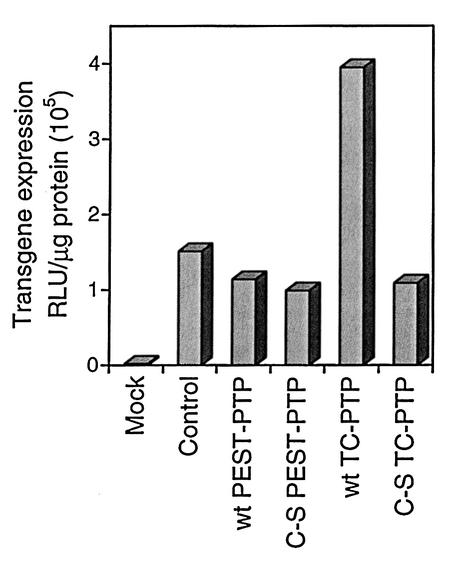
It was documented previously (36) that inhibition of AAV second-strand DNA synthesis and, consequently, AAV-mediated transgene expression by FKBP52 that had been phosphorylated at tyrosine residues was significantly higher than that at serine/threonine residues. Therefore, we set out in this study to identify the cellular tyrosine phosphatase responsible for catalyzing dephosphorylation of FKBP52. We reasoned that since AAV DNA synthesis occurs in the nucleus, tyrosine dephosphorylation of FKBP52 bound to AAV D sequence might also occur in the nucleus, which would be carried out by a protein tyrosine phosphatase present in the nucleus. One such candidate enzyme is TC-PTP (21, 47). We hypothesized that if FKBP52 is indeed dephosphorylated at tyrosine residues by TC-PTP, then deliberate overexpression of TC-PTP would lead to an increase in AAV transduction efficiency in HeLa cells. These cells contain FKBP52 that has been phosphorylated predominantly at tyrosine residues; although the cells are highly susceptible to AAV infection, they are poorly transduced by AAV vectors (34). Recombinant expression plasmids containing the Rous sarcoma virus (RSV) promoter-driven murine TC-PTP cDNA, either the wt TC-PTP or a mutant in which the catalytic cysteine residue in the active site had been replaced with a serine residue (C-S mutant TC-PTP), were used to stably transfect HeLa cells. Expression plasmids containing either the wt or the C-S mutant of an additional protein tyrosine phosphatase, PEST-PTP (1, 5), were also included in these experiments. TC-PTP gene expression was analyzed by Northern blotting and could be readily detected in transfected HeLa cells, whereas there was minimal expression of the endogenous gene in untransfected, control HeLa cells (data not shown). Highly purified stocks of a recombinant AAV vector containing the β-galactosidase (lacZ) reporter gene driven by the cytomegalovirus (CMV) immediate-early promoter (vCMVp-lacZ) were generated as described previously (32, 33). Approximately equivalent numbers of untransfected HeLa cells or HeLa cells stably transfected with wt or C-S mutant TC-PTP or PEST-PTP expression plasmids were infected at 37°C for 2 h with 5 × 103 particles per cell of the vCMVp-lacZ vector and the β-galactosidase activity was measured 48 h postinfection as previously described (12, 36). The results are shown in Fig. 1. As can be seen, although AAV transduction efficiency in HeLa cells stably transfected with either the wt or the C-S mutant PEST-PTP was not significantly different from that in untransfected HeLa cells, a nearly fourfold increase in AAV transduction efficiency in cells stably transfected with the wt TC-PTP expression plasmid was obtained. This increase was not seen when the C-S mutant TC-PTP expression plasmid was used. These results suggest that TC-PTP catalyzes tyrosine dephosphorylation of FKBP52, thereby leading to a lack of inhibition in viral second-strand DNA synthesis and increased transgene expression. These possibilities were tested experimentally as follows.
FIG. 1.
Comparative analyses of AAV-mediated transduction efficiency in HeLa cells stably transfected with wt and C-S mutant PEST-PTP or TC-PTP expression plasmids. Mock-transfected (Mock) or transfected HeLa cells were either mock infected (Control) or infected with a recombinant AAV-lacZ vector under identical conditions. Transgene expression was evaluated 48 h postinfection. These data, expressed as relative light units (RLU) per microgram of total protein, were within the linear range of the assay and represent average values from two separate experiments performed in triplicate.
TC-PTP catalyzes tyrosine dephosphorylation of FKBP52.
We wished to directly examine the effect of the deliberate expression of TC-PTP on the tyrosine phosphorylation status of FKBP52 by using electrophoretic mobility-shift assays (EMSAs). Whole cell extracts (WCE) from untransfected HeLa cells and those from wt and C-S mutant PEST-PTP- and TC-PTP-transfected cells were prepared according to the method described by Muller (27). Total protein concentration was determined by the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, Calif.), and the extracts were frozen in liquid N2 and stored at −70°C. EMSAs were performed as described previously (36, 37). Briefly, DNA-binding reactions were performed in a volume of 20 μl with 2 μg of poly(dI)-poly(dC), 2 μg of bovine serum albumin, and 12% glycerol in HEPES buffer (pH 7.9). Ten micrograms of protein from each WCE was preincubated for 10 min at 25°C followed by the addition of 10,000 cpm of 32P-labeled D sequence synthetic oligonucleotide (5′-AGGAACCCCTAGTGATGGAG-3′) in the reaction mixture. The binding reaction was allowed to proceed for 30 min at 25°C. Bound complexes were separated from the unbound probe on low-ionic strength 4% polyacrylamide gels using Tris-glycine-EDTA buffer (pH 8.5) containing 50 mM Tris-HCl, 380 mM glycine, and 2 mM EDTA. Following electrophoresis, the gel was dried in vacuuo and autoradiographed with Kodak X-Omat film at −70°C. The results are shown in Fig. 2. As can be seen, the AAV D sequence probe (lane 1) formed a complex with the tyrosine-phosphorylated form of FKBP52 in untransfected HeLa cells (lane 2), consistent with previously published results (36). Similar complexes were detected in WCE prepared from HeLa cells stably transfected with either the wt (lane 3) or the C-S mutant (lane 4) PEST-PTP expression plasmid. Interestingly, in WCE prepared from HeLa cells stably transfected with the wt TC-PTP expression plasmid (lane 5), the complex migrated faster, consistent with that of the tyrosine-dephosphorylated form of FKBP52. This mobility shift was not observed with WCE prepared from HeLa cells that had been stably transfected with the C-S mutant TC-PTP expression plasmid (lane 6). These results corroborate the hypothesis that tyrosine-phosphorylated FKBP52 is a substrate for TC-PTP.
FIG. 2.
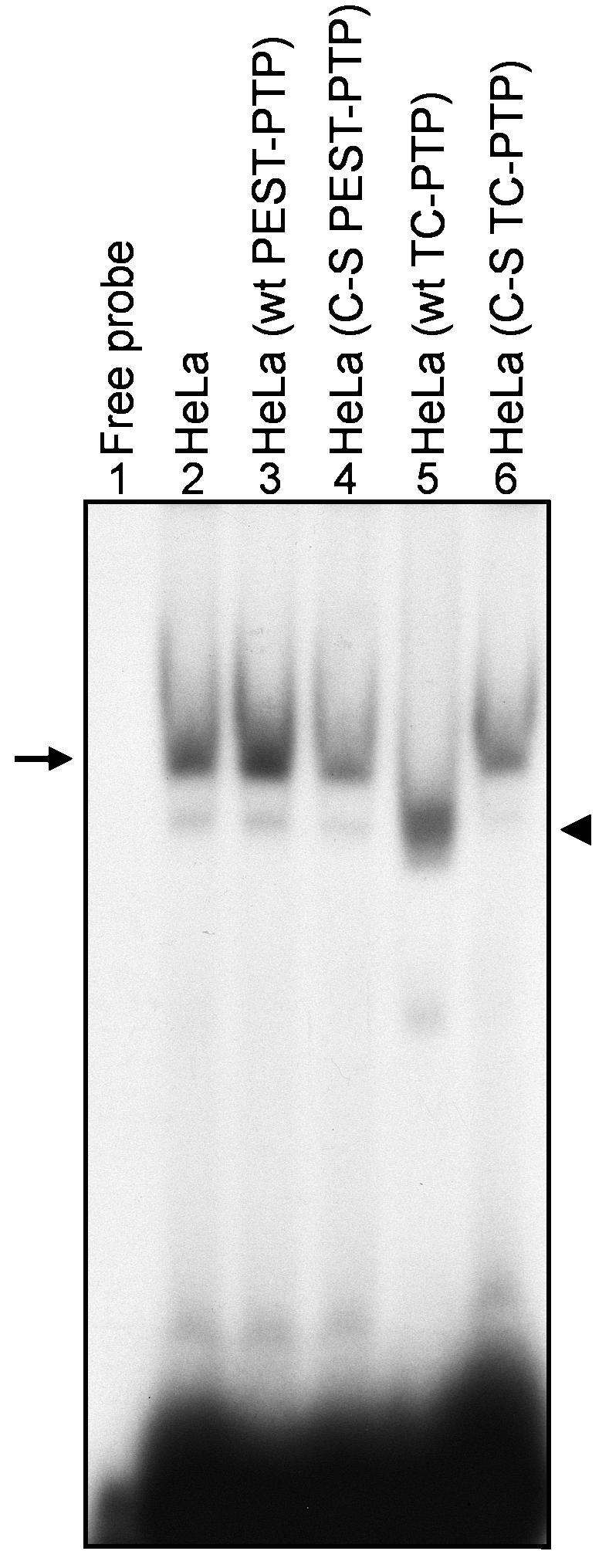
EMSAs for the tyrosine-phosphorylation status of FKBP52 in HeLa cells stably transfected with wt or C-S mutant PEST-PTP or TC-PTP expression plasmids. Tyrosine-phosphorylated and dephosphorylated forms of FKBP52 are denoted by the arrow and the arrowhead, respectively.
TC-PTP expression leads to increased AAV second-strand DNA synthesis.
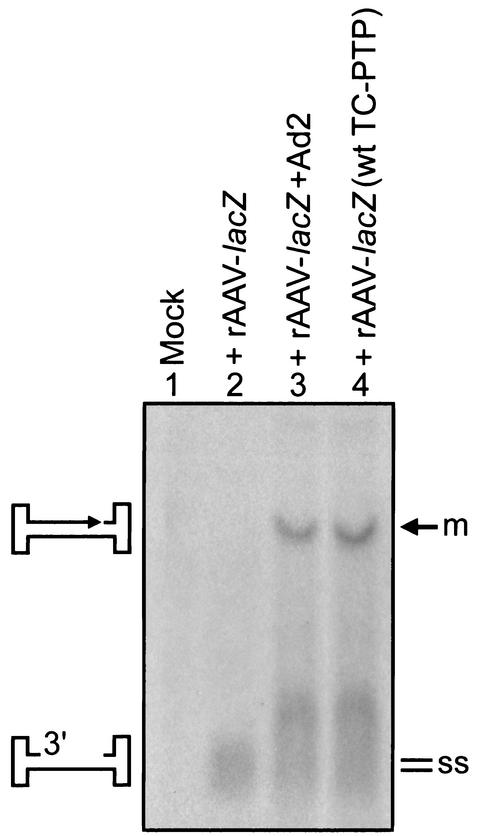
We next examined the effect of deliberate expression of the wt and the C-S mutant TC-PTP on the extent of AAV second-strand DNA synthesis. These assays were performed by infecting HeLa cells that were either not transfected or stably transfected with the wt or the C-S mutant TC-PTP expression plasmid or with a recombinant AAV-lacZ vector as described above. HeLa cells coinfected with wt adenovirus type 2 (Ad2), which is known to significantly enhance AAV second-strand DNA synthesis (8, 9, 37), were also included as a positive control. Low-Mr DNA was isolated from these cells 48 h postinfection and analyzed on Southern blots by using a lacZ-specific DNA probe as previously described (12, 13). As shown in Fig. 3, whereas no signal was detected in mock-infected HeLa cells (lane 1), major amounts of input genomic AAV vector were present as single strands in untransfected HeLa cells (lane 2), consistent with the observed low transduction efficiency of recombinant AAV vectors in these cells (12, 13, 37). In the presence of coinfection with Ad2 (lane 3), most of the viral genomes were converted to DNA duplex forms, presumably having undergone second-strand DNA synthesis consistent with their transcriptional activity (12, 13, 37). Interestingly, the input viral genomes were also readily converted into their duplex counterparts after second-strand DNA synthesis in HeLa cells stably transfected with the wt TC-PTP expression plasmid (lane 4). This effect was not seen in HeLa cells transfected with the C-S mutant TC-PTP expression plasmid (data not shown). Thus, TC-PTP-mediated tyrosine dephosphorylation of FKBP52 leads to efficient AAV second-strand DNA synthesis and results in an increase in AAV-mediated transgene expression.
FIG. 3.
Southern blot analysis of AAV second-strand DNA synthesis in HeLa cells coinfected with the wt adenovirus or stably transfected with wt TC-PTP expression plasmid. The input viral single-stranded DNA genomes and their monomer duplex counterparts are denoted by ss and m, respectively, on the right and schematically depicted on the left.
Primary murine hematopoietic stem/progenitor cells from TC-PTP-transgenic mice can be successfully transduced by recombinant AAV vectors.
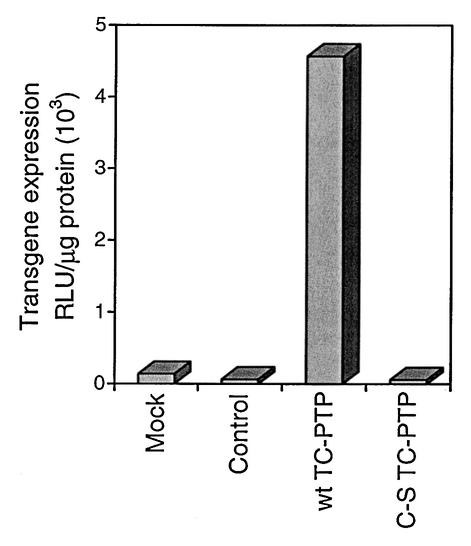
In order to examine the role of TC-PTP-mediated tyrosine dephosphorylation of FKBP52 on AAV-mediated transgene expression in vivo, we also generated transgenic mice expressing the murine wt or the C-S mutant TC-PTP gene, using normal C3HeB/FeJ mice. TC-PTP-transgenic mice were identified by PCR amplification of genomic DNA samples from tail snips by using the RSV promoter-TC-PTP gene-specific primer pair (5′-CGGTTAAATGTGCACAGTACTGGCC-3′ and 5′-CTACAACGAGAAGGTGCGAGAGC-3′). Primitive Sca1+, lin− hematopoietic stem/progenitor cells were isolated from three nontransgenic, wt TC-PTP-, and C-S mutant TC-PTP-transgenic mice each as previously described (44) and infected with the recombinant AAV-lacZ vector under conditions identical to those described above. Transgene expression was determined 48 h postinfection. These results are shown in Fig. 4. It is evident that although AAV transduction efficiency in primary murine hematopoietic stem/progenitor cells from normal mice was low, as observed previously (34), cells from the wt TC-PTP-transgenic mice could be transduced by AAV vectors with significantly higher efficiency. Sca1+, lin− cells from the C-S mutant TC-PTP-transgenic mice, on the other hand, could not be transduced efficiently with the AAV vector. These studies further support our contention that TC-PTP-mediated tyrosine dephosphorylation of FKBP52 is a crucial determinant of AAV transduction efficiency.
FIG. 4.
Comparative analyses of AAV-mediated transduction efficiency in primary murine ScaI+, Lin− primitive hematopoietic stem/progenitor cells from nontransgenic and wt or C-S mutant TC-PTP-transgenic mice. Cells from nontransgenic mice were either mock infected (Mock) or infected (Control) with the recombinant AAV-lacZ vector under conditions identical to those for transgenic mice. The data are from cells obtained from three animals in each group and represent the average values from two separate experiments performed in triplicate.
It has become increasingly clear that AAV vectors have gained prominence as a useful alternative to the more commonly used retroviral and adenoviral vectors for human gene therapy (24). However, it has also become clear that AAV vectors encounter at least three major obstacles in certain cell types that negatively affect high-efficiency transduction by these vectors. These obstacles include (i) lack of optimal expression of the cellular receptor heparan sulfate proteoglycan HSPG for viral binding (43) and lack of optimal expression of the coreceptors fibroblast growth factor receptor 1 (FGFR1) and/or αVβ5 integrin for viral entry (35, 42), (ii) impaired endosomal processing leading to inefficient intracellular viral trafficking into the nucleus (6, 7, 12, 13), and (iii) the inability of AAV to synthesize its second-strand DNA to become transcriptionally active (8, 9) due to the presence of phosphorylated forms of FKBP52 (22, 23, 34, 36, 37). Of the three, it appeared that the last obstacle was most amenable to be overcome since in previous studies from our laboratory, we were able to modulate the phosphorylation status of FKBP52 to achieve high-efficiency transduction by AAV vectors by using specific inhibitors of cellular tyrosine kinases (22, 23, 34, 36, 37). However, more often than not, these inhibitors are cytotoxic to primary cells. This prompted us to explore the alternative by way of identification of the putative cellular tyrosine phosphatase responsible for catalyzing tyrosine dephosphorylation of FKBP52 in the hope of exploiting this enzyme to achieve the same objective. The potential involvement of a cellular tyrosine phosphatase in AAV-mediated transduction has previously been proposed (39). The identification of TC-PTP as the key protein tyrosine phosphatase in our present studies and its seemingly harmless deliberate expression in vitro and in vivo to significantly enhance AAV transduction efficiency both in established human cell lines and primary murine cells bode well for its eventual use in primary human cells. One such strategy we envisage is the use of recently described self-complementary AAV (scAAV) vectors (25) carrying the wt TC-PTP gene. These scAAV-TC-PTP vectors could be admixed with any conventional recombinant AAV vector prior to transduction. Under optimal conditions, transient expression of TC-PTP from the scAAV vector, which would not require viral second-strand DNA synthesis, would cause tyrosine dephosphorylation of FKBP52 which in turn would lead to more efficient second-strand DNA synthesis of the conventional AAV vector, resulting in stable, high-efficiency transgene expression.
Although this attractive possibility remains to be tested experimentally, at the very least, the availability of TC-PTP-transgenic mice should allow us to gain further knowledge of the role of this crucial protein tyrosine phosphatase in AAV-mediated gene transfer in a variety of different cell and tissue types, which should have important implications in the optimal use of recombinant AAV vectors in human gene therapy.
Acknowledgments
We thank Michel Tremblay for generously providing the PEST-PTP and TC-PTP expression plasmids and Jacqueline Hobbs for a critical review of the manuscript.
This research was supported in part by Public Health Service grants R01 HL-58881 and HL-65570 (to A.S.) and R01 HL-63169 (to M.C.Y.) from the National Institutes of Health. K.A.W.-K. was supported by postdoctoral training grant T32 HL-07910 from the NIH.
REFERENCES
- 1.Angers-Loustau, A., J. F. Cote, A. Charest, D. Dowbenko, S. Spencer, L. A. Lasky, and M. L. Tremblay. 1999. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J. Cell Biol. 144:1019-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berns, K. I., and C. Giraud. 1996. Biology of adeno-associated virus. Curr. Top. Microbiol. Immunol. 218:1-23. [DOI] [PubMed] [Google Scholar]
- 3.Carter, B. J., and T. R. Flotte. 1996. Development of adeno-associated virus vectors for gene therapy of cystic fibrosis. Curr. Top. Microbiol. Immunol. 218:119-144. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee, S., D. Lu, G. Podsakoff, and K. K. Wong, Jr. 1995. Strategies for efficient gene transfer into hematopoietic cells: the use of adeno-associated virus vectors in gene therapy. Ann. N. Y. Acad. Sci. 770:79-90. [DOI] [PubMed] [Google Scholar]
- 5.Cote, J. F., A. Charest, J. Wagner, and M. L. Tremblay. 1998. Combination of gene targeting and substrate trapping to identify substrates of protein tyrosine phosphatases using PTP-PEST as a model. Biochemistry 37:13128-13137. [DOI] [PubMed] [Google Scholar]
- 6.Douar, A.-M., K. Poulard, D. Stockholm, and O. Danos. 2001. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J. Virol. 75:1824-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan, D., Z. Yan, J. Yang, and J. F. Engelhardt. 2000. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Invest. 105:1573-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrari, F. K., T. Samulski, T. Shenk, and R. J. Samulski. 1996. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J. Virol. 70:3227-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher, K. J., G.-P. Gao, M. D. Weitzman, R. DeMatteo, J. F. Burda, and J. M. Wilson. 1996. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J. Virol. 70:520-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flotte, T. R., B. J. Carter, C. K. Conrad, W. B. Guggino, T. C. Reynolds, B. Rosenstein, G. Taylor, S. Walden, and R. Wetzel. 1996. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum. Gene Ther. 7:1145-1159. [DOI] [PubMed] [Google Scholar]
- 11.Flotte, T. R., S. A. Afione, C. Conrad, S. A. McGrath, R. Solow, H. Oka, P. L. Zeitlin, B. Guggino, and B. J. Carter. 1993. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc. Natl. Acad. Sci. USA 90:10613-10617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen, J., K. Y. Qing, H.-J. Kwon, C. Mah, and A. Srivastava. 2000. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J. Virol. 74:992-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen, J., K. Y. Qing, and A. Srivastava. 2001. Adeno-associated virus type 2-mediated gene transfer: altered endocytic processing enhances transduction efficiency in murine fibroblasts. J. Virol. 75:4080-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplitt, M. G., P. Leone, R. J. Samulski, X. Xiao, D. W. Pfaff, K. L. O'Malley, and M. J. During. 1994. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 8:148-153. [DOI] [PubMed] [Google Scholar]
- 15.Kaplitt, M. G., X. Xiao, R. J. Samulski, J. Li, K. Ojamaa, I. L. Klein, H. Makimura, M. J. Kaplitt, R. K. Strumpf, and E. B. Diethrich. 1996. Long-term gene transfer in porcine myocardium after coronary infusion of an adeno-associated virus vector. Ann. Thor. Surg. 62:1669-1676. [DOI] [PubMed] [Google Scholar]
- 16.Kay, M. A., C. S. Manno, M. V. Ragni, P. J. Larson, L. B. Couto, A. McClelland, B. Glader, A. J. Chew, S. J. Tai, R. W. Herzog, V. Arruda, F. Johnson, C. Scallan, E. Skarsgard, A. W. Flake, and K. A. High. 2000. Evidence for gene transfer and expression of factor IX in hemophilia B patients treated with an AAV vector. Nat. Genet. 28:257-261. [DOI] [PubMed] [Google Scholar]
- 17.Kessler, P. D., G. M. Podsakoff, X. Chen, S. A. McQuiston, P. C. Colosi, L. A. Matelis, G. J. Kurtzman, and B. J. Byrne. 1996. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. USA 93:14082-14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koeberl, D. D., I. E. Alexander, C. L. Halbert, D. W. Russell, and A. D. Miller. 1997. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc. Natl. Acad. Sci. USA 94:1426-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotin, R. M., J. C. Menninger, D. C. Ward, and K. I. Berns. 1991. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics 10:831-834. [DOI] [PubMed] [Google Scholar]
- 20.Kotin, R. M., M. Siniscalco, R. J. Samulski, X. D. Zhu, L. A. Hunter, C. A. Laughlin, S. K. McLaughlin, N. Muzyczka, M. Rocchi, and K. I. Berns. 1990. Site-specific integration by adeno-associated virus. Proc. Natl. Acad. Sci. USA 87:2211-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenzen, J. A., C. Y. Dadabay, and E. H. Fischer. 1995. COOH-terminal sequence motifs target the T cell protein tyrosine phosphatase to the ER and nucleus. J. Biol. Chem. 131:631-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mah, C., K. Y. Qing, J. Hansen, B. Khuntrirat, M. C. Yoder, and A. Srivastava. 1999. Gene transfer with adeno-associated virus 2 vectors: the growth factor receptor connection. Gene Ther. Mol. Biol. 3:57-65. [Google Scholar]
- 23.Mah, C., K. Y. Qing, B. Khuntrirat, S. Ponnazhagan, X.-S. Wang, D. M. Kube, M. C. Yoder, and A. Srivastava. 1998. Adeno-associated virus 2-mediated gene transfer: role of epidermal growth factor receptor protein tyrosine kinase in transgene expression. J. Virol. 72:9835-9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall, E. 2001. Viral vectors still pack surprises. Science 294:1640.. [DOI] [PubMed] [Google Scholar]
- 25.McCarty, D. M., P. E. Monahan, and R. J. Samulski. 2001. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 8:1248-1254. [DOI] [PubMed] [Google Scholar]
- 26.McCown, T. J., X. Xiao, J. Li, G. R. Breese, and R. J. Samulski. 1996. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 713:99-107. [DOI] [PubMed] [Google Scholar]
- 27.Muller, M. T. 1987. Binding of herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J. Virol. 61:858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muzyczka, N. 1992. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 158:97-129. [DOI] [PubMed] [Google Scholar]
- 29.Nathwani, A. C., H. Hanawa, J. Vandergriff, P. Kelly, E. F. Vanin, and A. W. Nienhuis. 2000. Efficient gene transfer into human cord blood CD34+ cells and the CD34+CD38− subset using highly purified recombinant adeno-associated viral vector preparations that are free of helper virus and wild-type AAV. Gene Ther. 7:183-195. [DOI] [PubMed] [Google Scholar]
- 30.Ping, P., Q. Yang, and H. K. Hammond. 1996. Altered beta-adrenergic receptor signaling in heart failure: in vivo gene transfer via adeno and adeno-associated virus. Microcirculation 3:225-228. [DOI] [PubMed] [Google Scholar]
- 31.Ponnazhagan, S., P. Mukherjee, X.-S. Wang, K. Y. Qing, D. M. Kube, C. Mah, C. Kurpad, M. C. Yoder, E. F. Srour, and A. Srivastava. 1997. Adeno-associated virus type 2-mediated transduction of primary human bone marrow-derived CD34+ hematopoietic progenitor cells: donor variation and correlation of transgene expression with cellular differentiation. J. Virol. 71:8262-8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponnazhagan, S., P. Mukherjee, M. C. Yoder, X.-S. Wang, S. Z. Zhou, J. Kaplan, S. Wadsworth, and A. Srivastava. 1997. Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene 190:203-210. [DOI] [PubMed] [Google Scholar]
- 33.Ponnazhagan, S., M. C. Yoder, and A. Srivastava. 1997. Adeno-associated virus type 2-mediated transduction of murine hematopoietic cells with long-term repopulating ability and sustained expression of a human globin gene in vivo. J. Virol. 71:3098-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qing, K. Y., B. Khuntrirat, C. Mah, D. M. Kube, X.-S. Wang, S. Ponnazhagan, S. Z. Zhou, V. J. Dwarki, M. C. Yoder, and A. Srivastava. 1998. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J. Virol. 72:1593-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qing, K. Y., C. Mah, J. Hansen, S. Z. Zhou, V. J. Dwarki, and A. Srivastava. 1999. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 5:71-77. [DOI] [PubMed] [Google Scholar]
- 36.Qing, K. Y., J. Hansen, K. A. Weigel-Kelley, M. Q. Tan, S. Z. Zhou, and A. Srivastava. 2001. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J. Virol. 75:8968-8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qing, K. Y., X.-S. Wang, D. M. Kube, S. Ponnazhagan, A. Bajpai, and A. Srivastava. 1997. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc. Natl. Acad. Sci. USA 94:10879-10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samulski, R. J., X. Zhu, X. Xiao, J. D. Brook, D. E. Houseman, N. Epstein, and L. A. Hunter. 1991. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 10:3941-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanlioglu, S., and J. F. Englehardt. 1999. Cellular redox state alters recombinant adeno-associated virus transduction through tyrosine phosphatase pathways. Gene Ther. 6:1427-1437. [DOI] [PubMed] [Google Scholar]
- 40.Snyder, R. O., C. H. Miao, G. A. Patijn, S. K. Spratt, O. Danos, D. Nagy, A. M. Gown, B. Winther, L. Meuse, L. K. Cohen, A. R. Thompson, and M. A. Kay. 1997. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat. Genet. 16:270-276. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava, A., X.-S. Wang, S. Ponnazhagan, S. Z. Zhou, and M. C. Yoder. 1996. Adeno-associated virus 2-mediated transduction and erythroid lineage-specific expression in human hematopoietic progenitor cells. Curr. Top. Microbiol. Immunol. 218:93-117. [DOI] [PubMed] [Google Scholar]
- 42.Summerford, C., J. S. Bartlett, and R. J. Samulski. 1999. αVβ5 integrin: a co-receptor for adeno-associated virus 2 infection. Nat. Med. 5:78-82. [DOI] [PubMed] [Google Scholar]
- 43.Summerford, C., and R. J. Samulski. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan, M. Q., K. Y. Qing, S. Z. Zhou, M. C. Yoder, and A. Srivastava. 2001. Adeno-associated virus 2-mediated transduction and erythroid lineage-restricted, long-term expression of the human β-globin gene in hematopoietic cells from homozygous β-thalassemic mice. Mol. Ther. 3:940-946. [DOI] [PubMed] [Google Scholar]
- 45.Walsh, C. E., Liu. J. M., Miller, A. W. Nienhuis, and R. J. Samulski. 1993. Gene therapy for human hemoglobinopathies. Proc. Soc. Exp. Biol. Med. 204:289-300. [DOI] [PubMed] [Google Scholar]
- 46.Xiao, X., J. Li, and R. J. Samulski. 1996. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 70:8098-8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.You-Ten, K. E., E. Muise, A. Itie, E. Michaliszyn, J. Wagner, S. Jothy, W. S. Lapp, and M. L. Tremblay. 1997. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J. Exp. Med. 186:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou, S. Z., Q. Li, G. Stamatoyannopoulos, and A. Srivastava. 1996. Adeno-associated virus 2-mediated transduction and erythroid cell-specific expression of a human β-globin gene. Gene Ther. 3:223-229. [PubMed] [Google Scholar]