Abstract
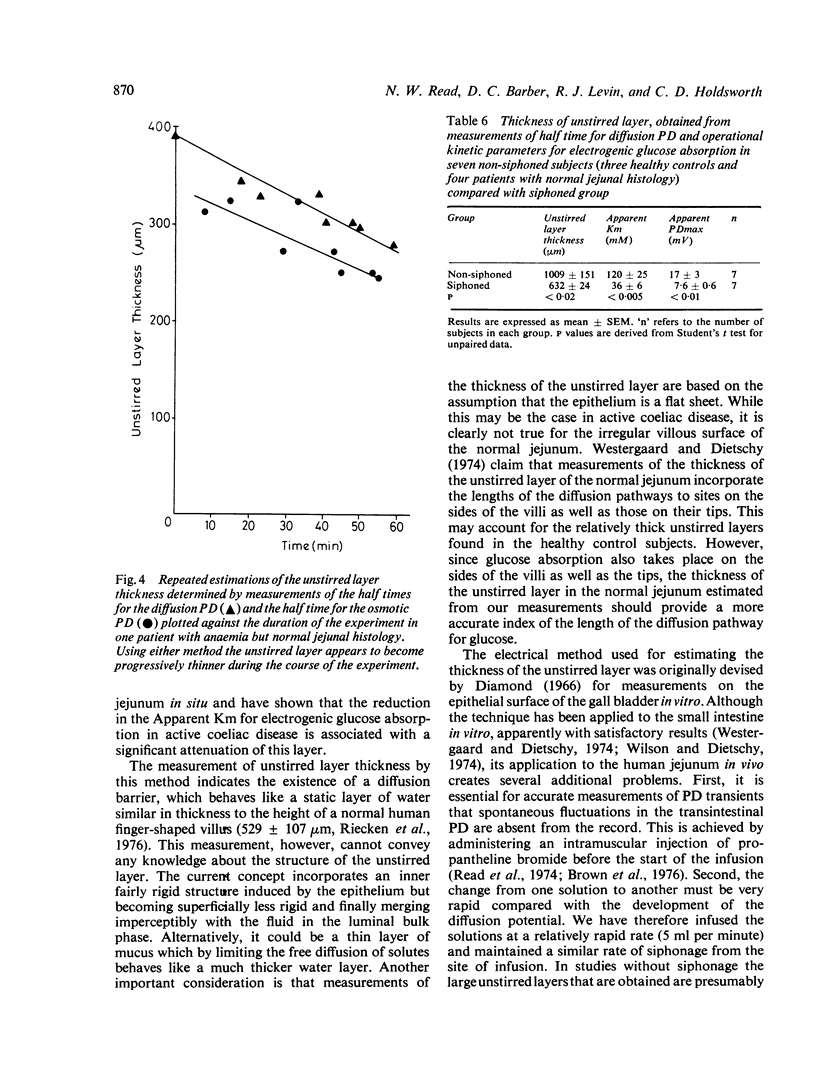
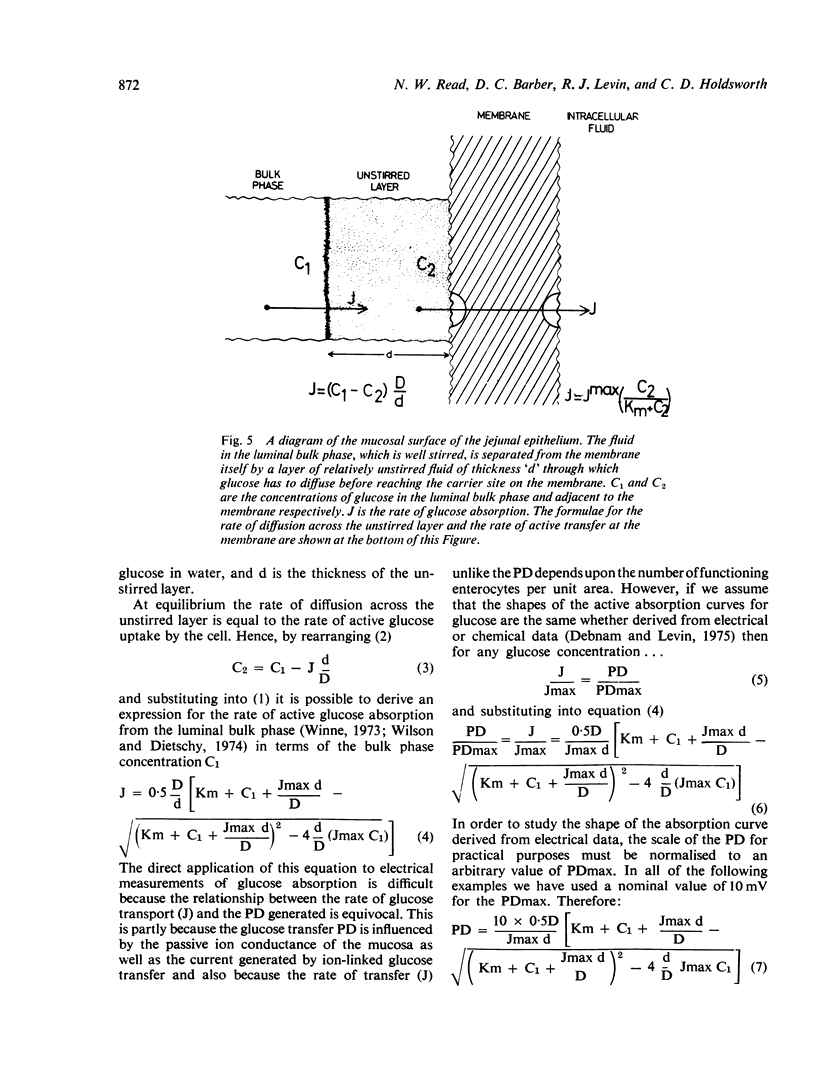
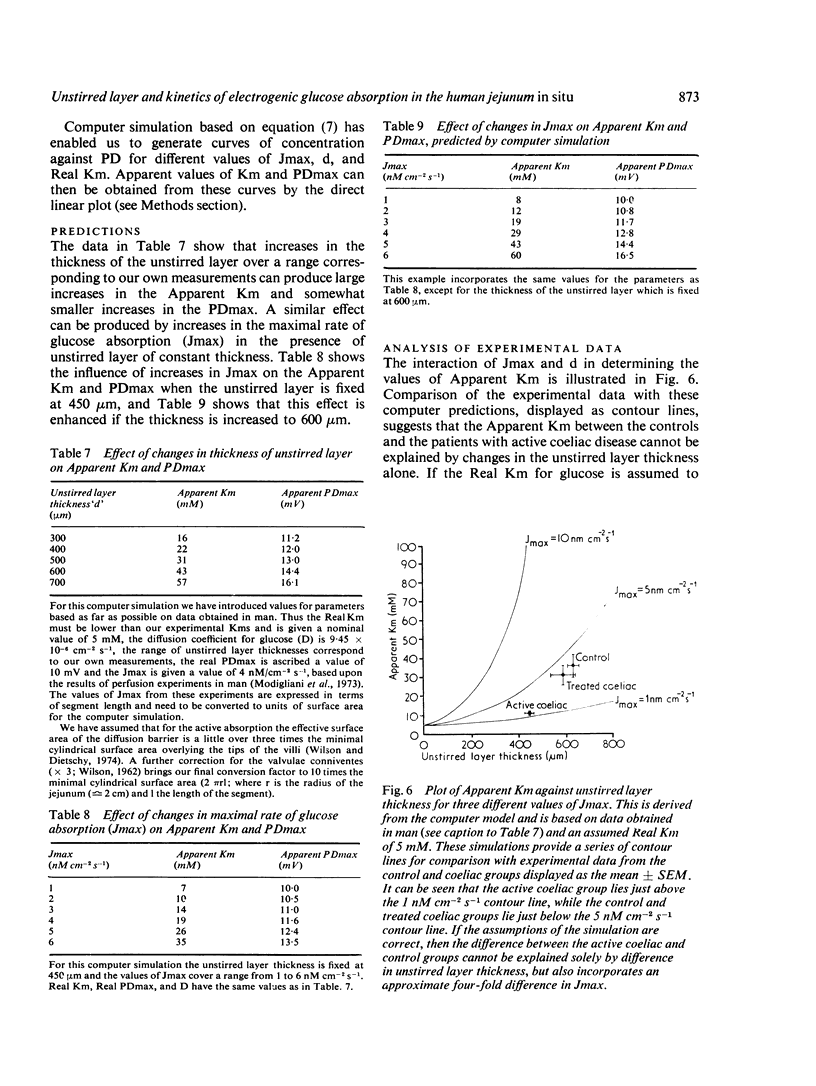
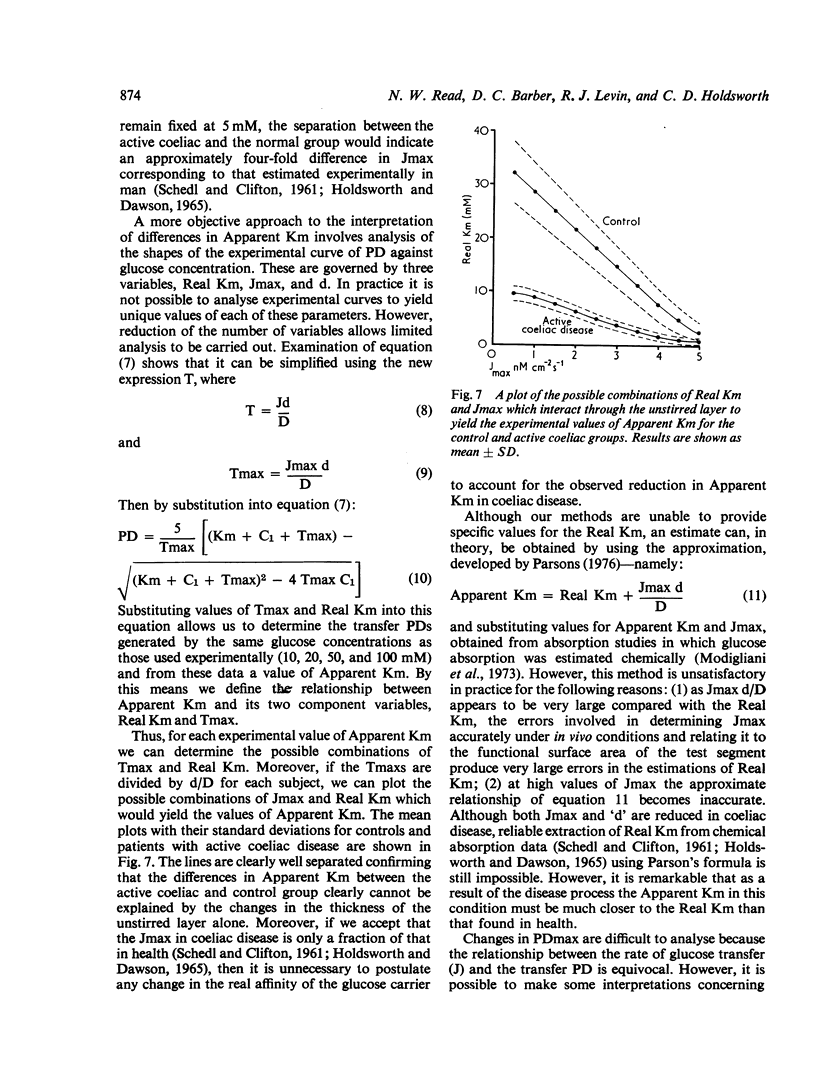
Using an electrical technique we estimated the thickness of the unstirred layer in the human jejunum during kinetic studies of electrogenic glucose absorption. The unstirred layer in seven healthy volunteers (632 ± 24 μm: mean ± SEM) was significantly thicker than in 10 patients with active coeliac disease (442 ± 23 μm) but not significantly different in seven patients who had responded to treatment by gluten withdrawal (585 ± 49 μm). There were similar differences in the values of `Apparent Km' for electrogenic glucose absorption between healthy control subjects (36 ± 6 mM) active coeliac patients (11 ± 1 mM) and treated coeliac patients (31 ± 5 mM). The changes in PDmax however, showed a different pattern. The PDmax in the active coeliac group (6·8 ± 0·7 mV) was lower than in controls (7·6 ± 0·6 mV) but not significantly so, while the PDmax in the treated coeliac group (10·6 ± 0·9 mV) was significantly higher than in both the active coeliac and control groups. It should be noted that both operational kinetic parameters obtained in the present study are much lower than those obtained previously (Read et al., 1976b) because of the use of siphonage. Analysis of the results using a computer simulation indicates that the reduction in Apparent Km in active coeliac disease can be caused by the interaction of the decreased maximal absorption rate for glucose (Jmax) with the attenuated unstirred layer. In these circumstances it is not necessary to postulate any change in the affinity of the transport mechanism for glucose (`Real Km'). It is remarkable that the disease process produces an Apparent Km which is much closer to the Real Km than that found in health.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown B. H., Holdsworth C. D., Levin R. J., Read N. W., Smallwood R. H. Proceedings: The relationship between intestinal motility and fluctuations in transmural potential difference in the human jejunum. J Physiol. 1976 Jul;259(1):29P–30P. [PubMed] [Google Scholar]
- CROSBY W. H., KUGLER H. W. Intraluminal biopsy of the small intestine; the intestinal biopsy capsule. Am J Dig Dis. 1957 May;2(5):236–241. doi: 10.1007/BF02231100. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Levin R. J. An experimental method of identifying and quantifying the active transfer electrogenic component from the diffusive component during sugar absorption measured in vivo. J Physiol. 1975 Mar;246(1):181–196. doi: 10.1113/jphysiol.1975.sp010885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M. A rapid method for determining voltage-concentration relations across membranes. J Physiol. 1966 Mar;183(1):83–100. doi: 10.1113/jphysiol.1966.sp007852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Sallee V. L., Wilson F. A. Unstirred water layers and absorption across the intestinal mucosa. Gastroenterology. 1971 Dec;61(6):932–934. [PubMed] [Google Scholar]
- Dugas M. C., Ramaswamy K., Crane R. K. An analysis of the D-glucose influx kinetics of in vitro hamster jejunum, based on considerations of the mass-transfer coefficient. Biochim Biophys Acta. 1975 Apr 8;382(4):576–589. doi: 10.1016/0005-2736(75)90224-2. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER R. B., PARSONS D. S. Glucose movements across the wall of the rat small intestine. J Physiol. 1953 Feb 27;119(2-3):210–223. doi: 10.1113/jphysiol.1953.sp004839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth C. D., Dawson A. M. Glucose and fructose absorption in idiopathic steatorrhoea. Gut. 1965 Aug;6(4):387–391. doi: 10.1136/gut.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modigliani R., Rambaud J. C., Bernier J. J. The method of intraluminal perfusion of the human small intestine. II. Absorption studies in health. Digestion. 1973 Oct;9(3):264–290. doi: 10.1159/000197453. [DOI] [PubMed] [Google Scholar]
- Read N. W., Holdsworth C. D., Levin R. J. Electrical measurement of intestinal absorption of glucose in man. Lancet. 1974 Sep 14;2(7881):624–627. doi: 10.1016/s0140-6736(74)91946-1. [DOI] [PubMed] [Google Scholar]
- Read N. W., Levin R. J., Holdsworth C. D. Electrogenic glucose absorption in untreated and treated coeliac disease. Gut. 1976 Jun;17(6):444–449. doi: 10.1136/gut.17.6.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read N. W., Levin R. J., Holdsworth C. D. Proceedings: Measurement of the functional unstirred layer thickness in the human jejunum in vivo. Gut. 1976 May;17(5):387–387. [PubMed] [Google Scholar]
- Thomson A. B., Dietschy J. M. Derivation of the equations that describe the effects of unstirred water layers on the kinetic parameters of active transport processes in the intestine. J Theor Biol. 1977 Jan 21;64(2):277–294. doi: 10.1016/0022-5193(77)90357-5. [DOI] [PubMed] [Google Scholar]
- Westergaard H., Dietschy J. M. Delineation of the dimensions and permeability characteristics of the two major diffusion barriers to passive mucosal uptake in the rabbit intestine. J Clin Invest. 1974 Sep;54(3):718–732. doi: 10.1172/JCI107810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. A., Dietschy J. M. The intestinal unstirred layer: its surface area and effect on active transport kinetics. Biochim Biophys Acta. 1974 Aug 21;363(1):112–126. doi: 10.1016/0005-2736(74)90010-8. [DOI] [PubMed] [Google Scholar]
- Winne D. Unstirred layer thickness in perfused rat jejunum in vivo. Experientia. 1976 Oct 15;32(10):1278–1279. doi: 10.1007/BF01953092. [DOI] [PubMed] [Google Scholar]
- Winne D. Unstirred layer, source of biased Michaelis constant in membrane transport. Biochim Biophys Acta. 1973 Feb 27;298(1):27–31. doi: 10.1016/0005-2736(73)90005-9. [DOI] [PubMed] [Google Scholar]
- Wright E. M. Diffusion potentials across the small intestine. Nature. 1966 Oct 8;212(5058):189–190. doi: 10.1038/212189a0. [DOI] [PubMed] [Google Scholar]


