Abstract
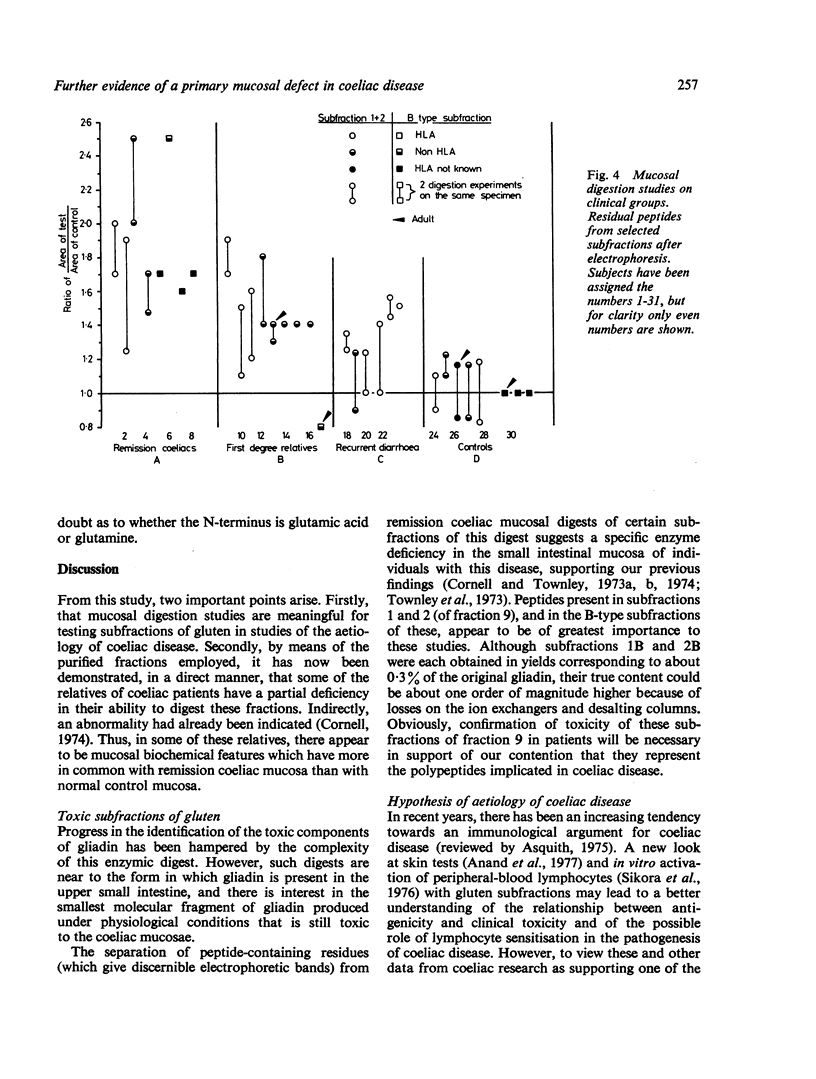
Subfractions of fraction 9, obtained from a peptic-tryptic-pancreatinic digest of wheat gliadin, were subjected to in vitro mucosal digestion and the filtrates examined for residual peptides. Small-intestinal mucosa from four groups of individuals were studied—eight patients with coeliac disease in remission; eight healthy controls; nine first degree relatives of patients with coeliac disease, and six children with recurrent diarrhoea investigated for possible coeliac disease, but in whom the diagnosis was excluded. The highest amounts of residual peptides (measured by scanning densitometer) were detected after digestion with mucosa from patients with coeliac disease and the lowest amounts with the control groups. The results obtained with the group of relatives fell between those of the coeliac disease and control groups, while the recurrent diarrhoea group overlapped the relatives and controls. The residual peptides were derived chiefly from the B-type subfractions of subfractions 1 and 2, obtained by ion-exchange chromatography of fraction 9. These subfractions are rich in glutamine/glutamic acid and proline and have a molecular weight (apparent) of not greater than 1500 Daltons. The results lend further support to the hypothesis of an enzyme deficiency in coeliac disease. A partial enzyme deficiency may exist in some first-degree relatives and in some children with recurrent diarrhoea but with histology of the small intestine within normal limits. HLA-B8 antigen is not correlated with this deficiency, but, when the two factors are associated, they could be related to the manifestation and severity of coeliac disease.
Full text
PDF
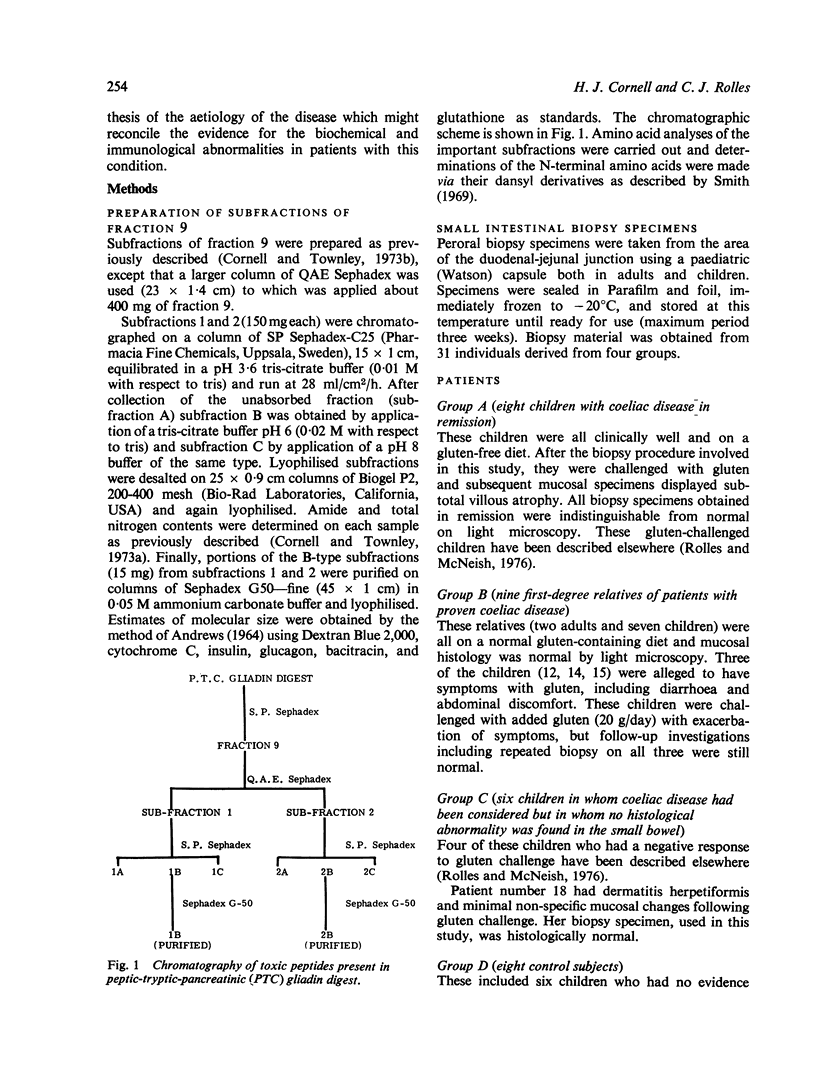
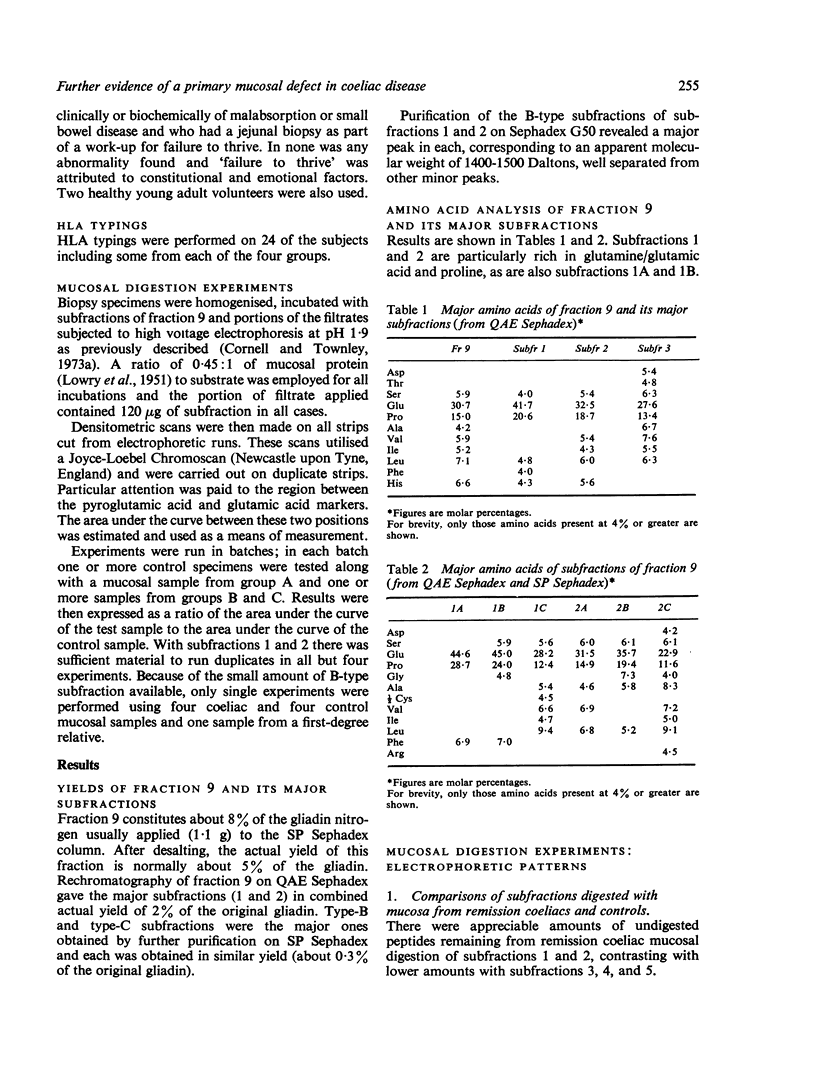
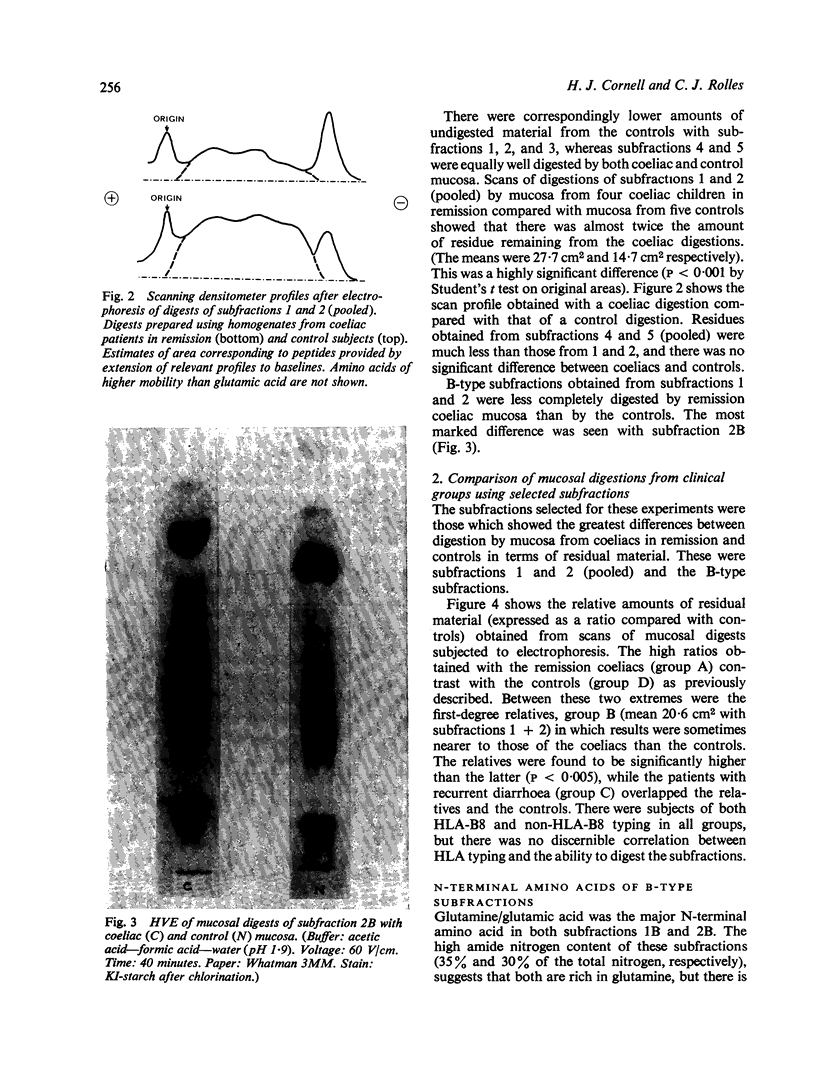


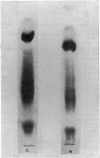
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand B. S., Truelove S. C., Offord R. E. Skin test for coeliac disease using a subfraction of gluten. Lancet. 1977 Jan 15;1(8003):118–120. doi: 10.1016/s0140-6736(77)91706-8. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein H. D., Haeffner L. J., Kowlessar O. D. Enzymatic digestion of gliadin: the effect of the resultant peptides in adult celiac disease. Clin Chim Acta. 1966 Aug;14(2):141–155. doi: 10.1016/0009-8981(66)90080-5. [DOI] [PubMed] [Google Scholar]
- Cornell H. J. Circulating antibodies to wheat gliadin fractions in coeliac disease. Arch Dis Child. 1974 Jun;49(6):454–458. doi: 10.1136/adc.49.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell H. J., Townley R. R. Investigation of possible intestinal peptidase deficiency in coeliac disease. Clin Chim Acta. 1973 Jan 10;43(1):113–125. doi: 10.1016/0009-8981(73)90126-5. [DOI] [PubMed] [Google Scholar]
- Cornell H. J., Townley R. R. The effect of gliadin peptides on rat-liver lysosomes in relation to the pathogenesis of coeliac disease. Clin Chim Acta. 1973 Dec 12;49(2):181–188. doi: 10.1016/0009-8981(73)90289-1. [DOI] [PubMed] [Google Scholar]
- Cornell H. J., Townley R. R. The toxicity of certain cereal proteins in coeliac disease. Gut. 1974 Nov;15(11):862–869. doi: 10.1136/gut.15.11.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchuk Z. M., Rogentine G. N., Strober W. Predominance of histocompatibility antigen HL-A8 in patients with gluten-sensitive enteropathy. J Clin Invest. 1972 Jun;51(6):1602–1605. doi: 10.1172/JCI106958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson D. A., Cornell H. J., Purdham D. R., Rolles C. J. Non-specific cytotoxicity of wheat gliadin components towards cultured human cells. Lancet. 1976 Feb 14;1(7955):339–341. doi: 10.1016/s0140-6736(76)90089-1. [DOI] [PubMed] [Google Scholar]
- Keuning J. J., Peña A. S., van Leeuwen A., van Hooff J. P., va Rood J. J. HLA-DW3 associated with coeliac disease. Lancet. 1976 Mar 6;1(7958):506–508. doi: 10.1016/s0140-6736(76)90294-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McNeish A. S., Rolles C. J., Arthur L. J. Criteria for diagnosis of temporary gluten intolerance. Arch Dis Child. 1976 Apr;51(4):275–278. doi: 10.1136/adc.51.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolles C. J., McNeish A. S. Standardised approach to gluten challenge in diagnosing childhood coeliac disease. Br Med J. 1976 May 29;1(6021):1309–1311. doi: 10.1136/bmj.1.6021.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora K., Anand B. S., Truelove S. C., Ciclitira P. J., Offord R. E. Stimulation of lymphocytes from patients with coeliac disease by a subfraction of gluten. Lancet. 1976 Aug 21;2(7982):389–391. doi: 10.1016/s0140-6736(76)92406-5. [DOI] [PubMed] [Google Scholar]
- Stokes P. L., Asquith P., Holmes G. K., Mackintosh P., Cooke W. T. Histocompatibility antigens associated with adult coeliac disease. Lancet. 1972 Jul 22;2(7769):162–164. doi: 10.1016/s0140-6736(72)91330-x. [DOI] [PubMed] [Google Scholar]
- Townley R. R., Cornell H. J., Bhathal P. S., Mitchell J. D. Toxicity of wheat gliadin fractions in coeliac disease. Lancet. 1973 Jun 16;1(7816):1363–1364. doi: 10.1016/s0140-6736(73)91679-6. [DOI] [PubMed] [Google Scholar]
- Weiser M. M., Douglas A. P. An alternative mechanism for gluten toxicity in coeliac disease. Lancet. 1976 Mar 13;1(7959):567–569. doi: 10.1016/s0140-6736(76)90361-5. [DOI] [PubMed] [Google Scholar]