Abstract
Two maize genes with predicted translational similarity to the Arabidopsis FIE (Fertilization-Independent Endosperm) protein, a repressor of endosperm development in the absence of fertilization, were cloned and analyzed. Genomic sequences of fie1 and fie2 show significant homology within coding regions but none within introns or 5′ upstream. The fie1 gene is expressed exclusively in the endosperm of developing kernels starting at ∼6 days after pollination. fie1 is an imprinted gene showing no detectable expression of the paternally derived fie1 allele during kernel development. Conversely, fie2 is expressed in the embryo sac before pollination. After pollination, its expression persists, predominantly in the embryo and at lower levels in the endosperm. The paternal fie2 allele is not expressed early in kernel development, but its transcription is activated at 5 days after pollination. fie2 is likely to be a functional ortholog of the Arabidopsis FIE gene, whereas fie1 has evolved a distinct function. The maize FIE2 and sorghum FIE proteins form a monophyletic group, sharing a closer relationship to each other than to the FIE1 protein, suggesting that maize fie genes originated from two different ancestral genomes.
INTRODUCTION
The transition from quiescent ovule to proliferating zygote occurs after fertilization, which initiates seed development. In flowering plants, the ovule contains the female gametophyte that is composed of the egg and the central, synergid, and antipodal cells (Reiser and Fischer, 1993). Double fertilization stimulates the egg to develop into a diploid embryo and the central cell to develop into a triploid endosperm. In sexually reproducing plants, the embryo sac never develops into a seed without fertilization. However, in asexually reproducing apomictic plants, the egg cell develops parthenogenetically without fertilization to produce the embryo. In many species, termed nonautonomous apomicts, endosperm development still requires fertilization (Grimanelli et al., 2001; Koltunow, 2001). Significant progress toward understanding the genetic control of precocious seed development has been made in Arabidopsis. A number of mutants were isolated that uncouple seed development from fertilization and promote endosperm proliferation in the absence of fertilization. These mutants formed seed-like structures that were without embryos (Ohad et al., 1996; Chaudhury et al., 1997).
Three genes were identified that prevent fertilization-independent seed (FIS) development: FIS1/MEDEA, FIS2, and FIS3/FIE (Grossniklaus et al., 1998; Luo et al., 1999; Ohad et al., 1999). All three genes encode proteins related to Drosophila Polycomb group proteins, which are evolutionarily conserved and are considered to be transcriptional repressors in animals (Ng et al., 1997; Pirrotta, 1998). FIS1/MEDEA is related to the Polycomb group protein Enhancer of zeste (Grossniklaus et al., 1998; Luo et al., 1999), FIS2 is a C2H2 zinc finger protein with similarity to the Polycomb group protein Suppressor of zeste (Chaudhury et al., 2001), and FIS3/FIE is a homolog of the Polycomb protein ESC (Extra Sex Combs) (Ohad et al., 1999). ESC proteins belong to a family of WD-repeat proteins that promote protein–protein interaction in various multiprotein complexes (Sondek et al., 1996; Ng et al., 1997). Animal Polycomb protein complexes might repress transcription of their target genes by remodeling chromatin into a condensed inactive state (van der Vlag and Otte, 1999; Tie et al., 2001). In a similar manner, plant FIS complexes are thought to repress endosperm-promoting genes in the central cell of the embryo sac by chromatin-mediated silencing (Luo et al., 2000; Spillane et al., 2000). The target genes of the FIS complexes are not known, but they are likely to be key players in initiating developmental programs in the zygote.
Additionally, the FIS genes play a role in postfertilization seed development. They continue to be expressed in the embryo and endosperm after fertilization and may be involved in multiple aspects of seed development. As global negative regulators of transcription, the FIS/Polycomb complexes may control different pathways through the silencing of key genes. FIS genes appear to control the establishment of the anterior-posterior polar axis in the endosperm (Sorensen et al., 2001). The FIE gene is involved in one more pathway: the repression of floral homeotic gene transcription during embryo and seedling development (Kinoshita et al., 2001). These results suggest that the FIE protein, encoded by a single-copy gene in the Arabidopsis genome, may form distinct complexes in different plant tissues and help to repress several developmental programs.
FIE, FIS2, and MEDEA display nonequivalent expression of maternally and paternally transmitted alleles, a hallmark of genomic imprinting (Kinoshita et al., 1999; Luo et al., 2000; Grossniklaus et al., 2001). The maternal FIS alleles are essential, whereas the paternal FIS allele plays little or no role in seed development (Luo et al., 2000; Yadegari et al., 2000). Maternal control of seed development is a complex interaction between genetic and epigenetic factors that is not yet understood completely (Haig and Westoby, 1989; Vinkenoog et al., 2000; Chaudhury and Berger, 2001; Vinkenoog and Scott, 2001).
Evolutionary conservation of the Polycomb proteins in plant and animal embryogenesis justifies a candidate gene approach using Arabidopsis as a model to identify homologous genes and pathways in cereal crops such as maize. In a previous study, we showed that of the three putative FIS proteins in Arabidopsis, FIS3/FIE had the highest level of translational similarity to several maize ESTs in the PHI/DuPont maize EST database (Pioneer Hi-Bred International). Sequence analysis revealed that there are transcripts of two maize genes, fie1 and fie2 (described previously as ZmFie1 and ZmFie2) (Springer et al., 2002). The presence of two fie genes in the maize genome raises questions regarding their evolution and functional specialization. To address these questions, we have performed sequence analysis of genomic copies of both maize fie genes and investigated their expression patterns in ovules before and after fertilization. We found that fie1 and fie2 show a significant level of homology at the nucleotide level only between their exons, with no homology between their introns or 5′ regulatory sequences. Predictably, fie1 and fie2 display different expression patterns. With respect to imprinting, the genes show marked differences. The fie1 paternal allele is silenced permanently, whereas the fie2 paternal allele is not expressed early in kernel development but attains full expression at 10 days after pollination (DAP). Phylogenetic analysis of several plant FIE proteins shows that sorghum and maize FIE2 proteins are more related to each other than to the FIE1 protein.
RESULTS
Genomic Structure of fie Loci
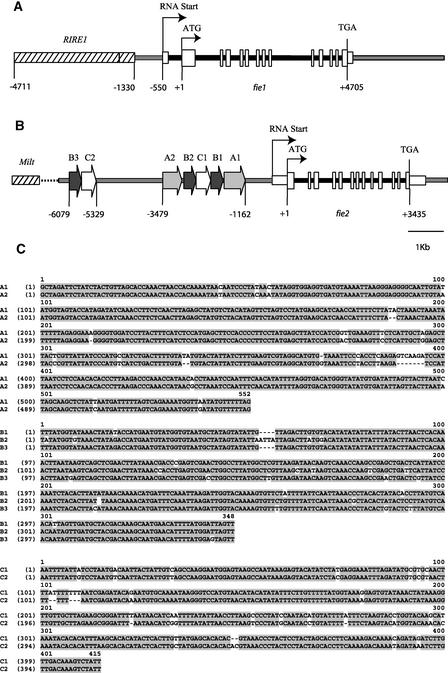
To determine the genomic structure of the two maize fie genes, their genomic DNAs were cloned using previously identified ESTs (Springer et al., 2002). A BAC library constructed from the public inbred line Mo17 was screened with gene-specific probes made from the 3′ untranslated region (UTR) of fie1 and fie2 (see Methods). Genomic fragments of ∼12 kb from each fie gene were subcloned and sequenced. The intron-exon structure for each locus was determined by aligning the genomic sequences with their corresponding cDNA sequences (Figures 1A and 1B).
Figure 1.
Genomic Structure of fie Loci.
Genomic segments (12 kb) of fie1 (chromosome 4, bin 4.05) (A) and fie2 (chromosome 10, bin 10.03) (B) are shown. Genes were mapped previously (Springer et al., 2002). The predicted start and stop codons of the fie coding regions are indicated by ATG and TGA, respectively. Positions of nucleotides are relative to the translation start codon ATG (+1). The putative transcription and translation start sites are shown as bent arrows; exons are shown as tall vertical boxes, UTRs as shorter boxes, and introns as thick dark lines. Regions that have homology with retrotransposons are stippled. The direct repeats positioned upstream of fie2 are marked by large arrows. (C) shows a sequence alignment of the A, B, and C repeats. Nucleotide identities are shaded.
The coding regions of both fie genes are composed of 13 exons ranging in length from 65 to 125 bp that are identical in size to each other and to the Arabidopsis FIE gene except for the first and last exons, where the initiation and termination of transcription occurs. The fie exonic sequences show 78% homology with each other. The coding sequences are interrupted by 12 introns, which vary in length from 63 to 1124 bp and show no detectable sequence similarity. fie1 has an additional 384-bp intron located in the 5′ UTR, just six nucleotides upstream of the ATG codon.
The putative promoter region of fie1 may be positioned in a 780-bp segment between the RNA transcription start (−550) and the long terminal repeat (−1330) of a RIRE retrotransposon. A 3.4-kb genomic fragment farther upstream (−1330 to −4711) is composed of several types of scrambled retrotransposons and MITE elements that have homology with genomic sequences at the bz2 locus (Fu et al., 2001).
The 5′ region of fie2 has a complex structure. A dot-plot alignment of the 6-kb sequence between the fie2 transcription start site and the first MILT retroelement has revealed a complex pattern of repeats (Figures 1B and 1C). Sequences between −1161 and −3479 consist of three types of repeats, named A, B, and C. These repeats form a 2.6-kb structure with the following symmetry: A1-B1-C1-B2-A2. The B3 and C2 repeats are positioned again at −5328 to −6077, forming another cluster. Repeats A1-A2 are 550 bp long and share 95% homology, B1-B2-B3 are 350 bp long with 94% homology, and C1-C2 are 420 bp long with 93% homology (Figure 1C). These repeats do not share any homology or features with any other known repetitive or transposable elements, as judged by a Basic Local Alignment Search Tool (BLAST) search of GenBank. These repeats are organized in a unique configuration that may be a potential cis-regulating element of fie2. We estimate the putative basal fie2 promoter to be ∼768 bp if framed between −393 and −1162. This marks the transcription start site of the longest EST identified to date and the beginning of repeat A1.
Expression of fie Genes in Developing Kernels
Preliminary data (Springer et al., 2002) indicated that fie1 and fie2 have different patterns of expression. fie1 mRNA was detected only in developing kernels, whereas fie2 expression was found in kernels and vegetative tissues. In this study, we applied different techniques, namely RNA gel blot analysis, gene expression analysis by massively parallel signature sequencing (MPSS) (Brenner et al., 2000a, 2000b), and reverse transcriptase–mediated (RT) PCR, to characterize the temporal and spatial expression patterns of both fie genes in developing kernels.
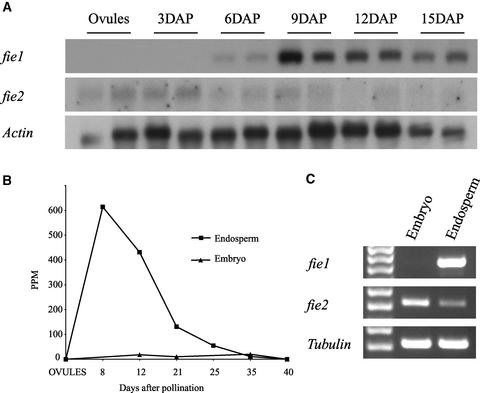
As shown on RNA gel blots (Figure 2A), fie1 mRNA is not detected in ovules or in 3-DAP zygotes. It appears first in 6-DAP kernels, reaching maximum accumulation at 9 DAP, and declines gradually in later stages. By contrast, fie2 mRNA is detected at very low level in ovules and at all stages of kernel development (Figure 2A).
Figure 2.
fie Expression in Developing Kernels.
(A) RNA gel blot analysis of mRNA isolated from ovules and kernels at 3, 6, 9, 12, and 15 DAP. A total of 3 μg of poly(A) RNA was loaded in each lane. Blots were probed with 32P-labeled 300-bp specific probes from the 3′ UTR of fie1 or fie2 cDNA (see Methods). Probes have no homology with each other and do not cross-hybridize. The actin probe was used as a loading control.
(B) Distribution of the 17-mer tags (5′-GATCTAGTGTGTGGCTG-3′) generated by MPSS from endosperm and embryo mRNAs. The vertical axis represents the frequency of tags as parts per million of molecules sequenced. The horizontal axis represents stages of kernel development starting with unfertilized ovules (point 0) and 8, 12, 21, 25, 35, and 40 DAP. The endosperms and embryos were dissected from kernels at 12 DAP and later stages. Squares and triangles indicate tag numbers in endosperms and embryos, respectively.
(C) RT-PCR of fie mRNA in the embryo and the endosperm isolated with a dissecting microscope from 16-DAP kernels. RT-PCR was performed with primers specific for fie1 or fie2. RT-PCR of α-tubulin cDNA was used as a positive control.
To achieve better spatial resolution and increased sensitivity, we queried an mRNA profiling database of different maize tissues created by MPSS technology at DuPont/Pioneer. MPSS generates 17-mer sequence tags of millions of cDNA molecules, which are cloned in vitro on microbeads (Brenner et al., 2000a, 2000b). The technique provides an unprecedented depth and sensitivity of mRNA detection, including messages expressed at very low levels. The MPSS profiling database is searchable by BLAST to identify gene-specific 17-mer tags. fie1-specific tags were not found in vegetative tissues, in the male reproductive tissues (tassel and mature pollen), or in female reproductive tissues (ovules). Tags were found only in developing kernels. The distribution of the fie1 tags is shown in Figure 2B. The numbers of tags found in the endosperm and embryo are significantly different, suggesting that fie1 is expressed predominantly in the endosperm. At 8 DAP, the number of fie1 tags approaches its maximum level of ∼600 ppm. The number of tags declines gradually to ∼20 ppm at 35 DAP and becomes undetectable at kernel maturity. This trend is consistent with the results of the mRNA gel blot experiment using whole kernels (Figure 2A). The MPSS technology requires the presence of a DpnII (GATC) restriction site in the cDNA template. Because fie2 lacks a 3′ DpnII site, there is no sequence tag information for fie2 in the MPSS database.
To further investigate fie expression in the embryo and endosperm, those tissues were dissected from 16-DAP kernels with precautions taken to avoid tissue cross-contamination. RT-PCR with gene-specific primers clearly demonstrates that fie1 is expressed exclusively in the endosperm, whereas fie2 is expressed predominantly in the embryo with lower levels of expression in the endosperm (Figure 2C). Thus, the two fie genes are expressed differentially in the embryo and endosperm of developing kernels.
In Situ Localization of fie2 Transcripts in Ovules and Developing Kernels
To localize fie2 expression within reproductive tissues, we performed RNA in situ hybridization. fie2 antisense probes gave a signal in the embryo sac of the mature ovules at silking (Figure 3A). No signal was detected in sporophytic tissues (i.e., the nucellus and pericarp), suggesting that fie2 is expressed specifically in the embryo sac. In kernels at 5 DAP (Figure 3B), an intense signal is apparent in the embryo, in the embryo-surrounding region, and on the periphery of the developing endosperm. At later stages, the signal persists in the embryo but is masked in the endosperm by starch grains that tend to show a reddish background (Figures 3C and 3D). By 15 DAP, the embryo is well developed and the vegetative apex, leaves, embryonic axis, coleoptiles, and radicle can be distinguished. There is a signal in all of these organs, but not in the scutellum (Figure 3C). The in situ hybridization results are consistent with our RNA gel blot and RT-PCR findings with the dissected embryo and endosperm. The results indicate that fie2 is expressed in ovules before pollination and in developing kernels after fertilization, predominantly in the embryo.
Figure 3.
Detection of fie2 mRNA in Ovules and Kernels by in Situ Hybridization.
Longitudinal sections of ovules and kernels at 5 and 15 DAP (inbred line B73) were hybridized with fie2 sense and antisense RNA probes. Signal is brownish red. The sense fie2 RNA probe, which was used as a negative control, did not reveal any signal (data not shown).
(A) Ovule at silking (differential interference contrast microscopy image).
(B) Embryo in a 5-DAP kernel.
(C) Embryo in a 15-DAP kernel.
(D) Whole kernel at 15 DAP.
EM, embryo; EN, endosperm; ES, embryo sac; N, nucellus; P, pericarp; S, scutellum. Bars = 0.1 mm in (A) to (C) and 1 mm in (D).
Pattern of Maternal and Paternal fie Allele Expression during Kernel Development
The Arabidopsis FIE gene demonstrates a parent-of-origin effect on seed development, suggesting that only the maternal FIE allele is essential and the paternal FIE allele plays little or no role in seed development (Luo et al., 2000; Yadegari et al., 2000). To understand whether the maize fie homologs are regulated in a similar manner, paternal and maternal fie mRNA levels were measured in developing kernels. To distinguish between maternal and paternal fie mRNAs, insertion/deletion sequence polymorphisms were identified for both fie1 and fie2 in the inbred lines Mo17 and B73.
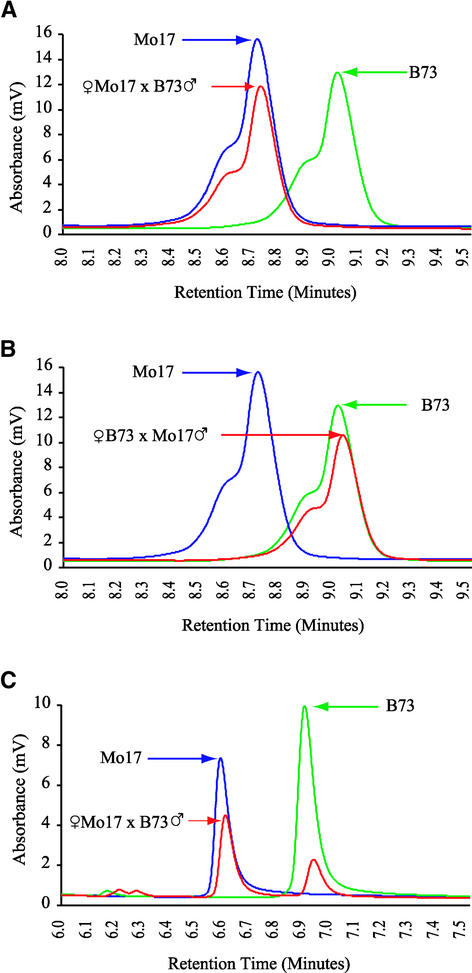
The fie1 Mo17 allele differs from the B73 allele by a 12-bp deletion located 96 bp upstream of the TGA stop codon. This deletion should remove four amino acids at the C-terminal end of the FIE1 protein in Mo17. Reverse and forward primers were designed around this insertion/deletion to produce a 300-bp RT-PCR product. Kernels were collected at 2, 5, 10, 15, and 16 DAP from plants of reciprocal crosses between B73 and Mo17 inbred lines. RT-PCR was performed, and fragments were separated on denaturing HPLC columns using the WAVE system (Transgenomics, Omaha, NE). The results for 15-DAP kernels from the reciprocal crosses are shown in Figures 4A and 4B. No expression of the fie1 paternal allele is detected; only the maternal fie1 RNA is found. The results are the same for all of the developmental stages from 2 to 15 DAP (results for early stages are not shown). This experiment demonstrates transcriptional silencing of the paternal fie1 allele, which suggests that the fie1 gene is regulated by imprinting. A control experiment for biallelic gene expression was performed with the same RNA samples. RT-PCR was performed with primers designed around a B73/Mo17 insertion/deletion in a novel gene. The expression of both paternal and maternal alleles was detected in 15-DAP kernels (Figure 4C).
Figure 4.
Paternal and Maternal fie1 mRNA Accumulation in Developing Kernels.
Graphs represent the size-dependent separation of RT-PCR amplification products by the WAVE denaturing HPLC system. Larger fragments are retained longer on the DNASEP cartridges, resulting in an accurate quantitative separation of fragments from a complex mixture. Total RNA was isolated from 15-DAP kernels from Mo17 × B73 reciprocal crosses. Unfertilized ovules and 11-DAP kernels from self-pollinated plants were sampled from both inbred lines as controls. Total RNA was extracted from whole kernels, and RT-PCR was performed with primers positioned around a 12-bp deletion in the 3′ UTR of the Mo17 allele.
(A) Expression of the maternal Mo17 allele in the Mo17 × B73 cross.
(B) Expression of the maternal B73 allele in a reciprocal cross.
(C) Biallelic expression of a control gene detected in the same RNA samples. The ratio of maternal to paternal peaks is 2.1:1. RT-PCR primers were designed around an insertion/deletion in the EST sequence MEST80-E04.T3 (courtesy of Mei Guo, Pioneer Hi-Bred International).
To determine whether imprinting of fie1 is specific to only the B73 and Mo17 alleles or if it reflects a general regulation of this locus, we performed the same set of experiments using SSS1 and NSS1, two Pioneer inbred lines. Paternally derived fie1 alleles are silenced in these backgrounds as well (data not shown). Thus, the ability of fie1 to undergo imprinting is a property of the fie1 locus itself and not the property of a specific allele.
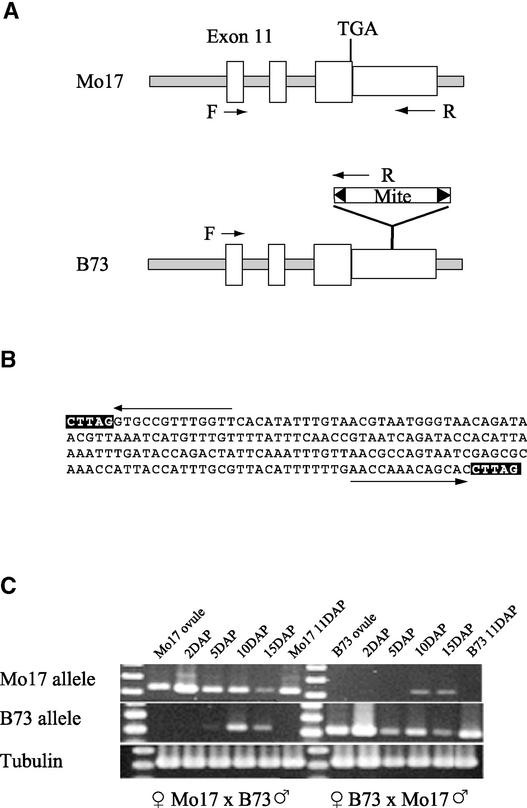
To investigate whether fie2 paternal and maternal allele expression differences exist, we exploited the fact that the fie2 B73 genomic sequence contains a 185-bp MITE insertion in the 3′ UTR that is not present in the Mo17 allele (Figure 5A). The insertion is flanked by 13-bp inverted repeats and a 5-bp direct target duplication (Figure 5B) similar to Tourist-like MITE elements (Wessler, 2001). The B73 fie2 poly(A) transcripts terminate in the middle of the MITE insertion. However, the Mo17 fie2 poly(A) transcripts terminate within the genomic sequence downstream of the MITE insertion site. The MITE sequence was used to design allele-specific primers to distinguish between B73 and Mo17 fie2 mRNAs (Figure 5A).
Figure 5.
Patterns of Paternal and Maternal fie2 mRNA Accumulation in Developing Kernels.
(A) fie2 Mo17 and B73 alleles are polymorphic as a result of a MITE insertion in the 3′ UTR in B73. Positions of a common forward primer (F) in exon 11 and genotype-specific reverse primers (R) in the 3′ UTR are indicated by arrows.
(B) DNA sequence of the 185-bp MITE insertion into the 3′ UTR of the fie2 B73 allele. The target-site duplications are boxed, and arrows mark the 13-bp terminal inverted repeats.
(C) Detection of maternal and paternal allele expression in developing kernels from reciprocal crosses. Reciprocal crosses between B73 and Mo17 were performed, and kernels were collected at 2, 5, 10, and 15 DAP. Total RNAs were isolated from whole kernels, including pericarp, and amplified by RT-PCR with the primers shown in (A). Ovules and 11-DAP selfed kernels were used as controls for primer specificity. α-Tubulin RT-PCR was used as a loading control.
The primer combinations are allele specific, as shown by RT-PCR of RNA isolated from both the ovules and the whole kernels of self-pollinated inbred plants (Figure 5C). fie2 maternal allele expression was detected at all stages in both reciprocal crosses. The maternally derived RT-PCR product shows increased intensity compared with ovules, suggesting de novo transcription of the maternal gene as early as 2 DAP. A similar pattern of expression also is seen in reciprocal crosses. The paternally derived RT-PCR product appears faintly in 5-DAP kernels but increases in intensity in 10- and 15-DAP kernels. Thus, the fie2 paternal allele shows delayed activation but not complete silencing. Delayed expression of the paternal allele also has been shown with reporter constructs for the Arabidopsis FIE (Luo et al., 2000; Yadegari et al., 2000).
The CG Composition of the fie Genes in Relation to Imprinting
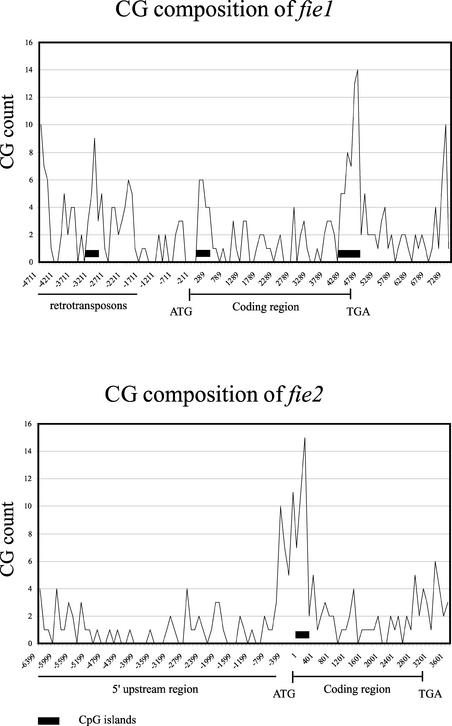
Differential CpG methylation has been shown to be a key molecular mechanism of imprinting in mammals (Wutz et al., 1997; Thorvaldsen et al., 1998; Reik and Walter, 2001). Recently, a two-island rule was proposed for genes regulated by imprinting (Onyango et al., 2000). According to this rule, the presence of two or more CpG islands is associated with imprinted genes, whereas the presence of one CpG island or none is associated with nonimprinted genes. CpG islands are defined as sequences of ∼200 bp with a GC content of >50% and an observed-to-expected CpG content of >60% (Gardiner-Garden and Frommer, 1987). We have computed the occurrence of CpG islands along the fie genomic sequences using the newcpgreport program available with the EMBOSS suite.
This analysis revealed three CpG islands within the fie1 locus. Two CpG islands are located within the fie1 region and one island is located in a retrotransposon segment between −2968 and −3219 (Figure 6, top). The first CpG island is 252 bp long and is positioned between +87 and +374, just downstream of the ATG codon (Figure 6, top). The second CpG island is 572 bp long and is positioned at the 3′ end of the gene, between +4315 and +4886, covering the last two introns and exons (Figure 6, top). Only one CpG island is present in the fie2 locus, between −231 and +88, near the ATG codon (Figure 6, bottom). fie CpG island distribution is consistent with the two-island rule for imprinted genes in mammals.
Figure 6.
Distribution of the CG Dinucleotides along fie Genomic Sequences.
Peaks represent the number of CG dinucleotides per 100 nucleotides. Start and stop codons are indicated by ATG and TGA, respectively. CpG islands are marked by closed rectangles.
Phylogenetic Analysis of Plant and Animal FIE/ESC Proteins
To gain insight into the evolutionary relationship between the maize fie genes, we performed phylogenetic analysis on FIE proteins from various plant species. Only Arabidopsis and maize FIE proteins are currently available in GenBank. Therefore, we identified FIE orthologs from four and five additional monocot and dicot species, respectively (Table 1), by searching the public EST databases using TBLASTX (Altschul et al., 1997). The FIE proteins belong to the WD Polycomb group proteins, which include the Drosophila ESC (Ng et al., 1997), the mouse Embryonic Ectoderm Development (EED) (Denisenko and Bomsztyk, 1997), the human WAIT (Rietzler et al., 1998), and the nematode MES6 (Korf et al., 1998) proteins. These also were included in the analysis. Three GBB (Guanine Nucleotide Binding Protein β-Subunit 2) WD-motif proteins (Sondek et al., 1996) were used as an outgroup.
Table 1.
Species Used for the Phylogenetic Analysis of FIE/ESC Proteins
| Latin Name | Common Name | Protein Name | GenBank Accession Numbers |
|---|---|---|---|
| Caenorhabditis elegans | Worm | MES6 | AF016224 |
| Mus musculus | Mouse | EED | U97675 |
| Homo sapiens | Human | WAIT-1 | AF078933 |
| Schistocerca americana | Grasshopper | ESC | AF003604 |
| Junonia coenia | Buckeye butterfly | ESC | AF003603 |
| Musca domestica | House fly | ESC | AF003605 |
| Drosophila virilis | Fruit fly | ESC | S80984 |
| Drosophila melanogaster | Fruit fly | ESC | L41867 |
| Arabidopsis thaliana | Thale cress | FIE | AF129516 |
| Hordeum vulgare | Barley | FIE |
BF256342 AV914549 |
| Triticum aestivum | Wheat | FIE |
BQ247234 BQ244502 |
| Oryza sativa | Rice | FIE |
C26788 AU164097 C26788 AU057691 BJ009205 |
| Zea mays | Maize | FIE1 | AY061964 |
| Zea mays | Maize | FIE2 | AY061965 |
| Sorghum bicolor | Sorghum | FIE |
BE362526 BG464362 BG488216 BE918589 AW677537 BG322850 |
| Glycine max | Soybean | FIE |
BI424774 BE211732 BM519641 BF598292 BI424788 |
| Eucalyptus grandis | Eucalyptus | FIE | AY150282 |
| Catalpa speciosa | Catalpa tree | FIE | AY150283 |
| Lycopersicon esculentum | Tomato | FIE |
BE353166 AW223682 BI923987 BG132016 |
| Solanum tuberosum | Potato | FIE |
BG594585 BG888279 BE919873 BE473138 |
| Homo sapiens | Human | GBB2 protein β2-subunit | p11016 |
| Mus musculus | Mouse | GBB4 transducin β chain 4 | p29387 |
| Drosophila melanogaster | Fruit fly | GBB2 | P29829 |
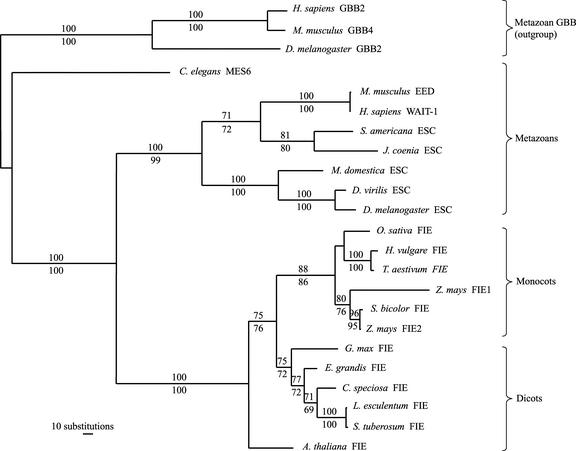
The neighbor-joining tree inferred using a distance matrix of a mean number of pair-wise character differences was topologically similar to the most parsimonious tree (Figure 7), with minor branch length differences. Phylogenetic trees reconstructed by the maximum-likelihood method also were topologically congruent to those constructed with the maximum-parsimony and neighbor-joining methods (data not shown).
Figure 7.
Phylogram of Plant and Animal FIE/ESC Proteins.
The Branch and Bound algorithm of the PAUP program found a single most parsimonious tree with a total length of 1913 steps. The numbers shown above and below the branches indicate the percentage of times a monophyletic group occurred among 500 bootstrap and jackknife replicates, respectively. Lengths of branches are proportional to the number of inferred amino acid substitutions. Species and protein names and accession numbers are listed in Table 1.
The phylogenetic tree clearly delimits four major clades that correspond to mammals, insects, monocots, and dicots, with the exception of Caenorhabditis elegans MES6 and Arabidopsis FIE proteins. Those proteins show a paraphyletic relationship to the other metazoan and dicot proteins, respectively. Both nodes were supported by high bootstrap and jackknife values (Figure 7). However, maximum-likelihood analysis indicated that the Arabidopsis FIE protein is monophyletic to the other dicot species (data not shown). Maize FIE2 and sorghum FIE proteins formed a monophyletic group by all of the methods used, strongly suggesting a closer relationship between them than between either one and the maize FIE1 protein.
DISCUSSION
Duplicated Maize fie Genes Arise from Different Ancestors
The presence of two types of fie genes in the maize genome raises questions about their origin, evolution, and functional specialization. Both genes share significant homology over their coding regions, and they have the same number of exons, all of which are identical in size except for the first and the last. However, the introns vary significantly in length and share no homology between fie1 and fie2 (Figure 1). The intron sequences are as variable between fie1 and fie2 as between both maize genes and the Arabidopsis FIE gene. The observation that the noncoding regions of the two maize fie genes diverged significantly is consistent with the idea that they evolved independently in two ancestral diploid species before the formation of the modern maize genome. This is in agreement with their locations on chromosome 4 (bin 4.05) for fie1 and on chromosome 10 (bin 10.03) for fie2, which are duplicated segments of the maize genome (Helentjaris, 1995). Recently duplicated genes often have similar exon/intron structures and retain significant (>90%) sequence similarity between their introns, as has been shown for the duplicated maize p1 and p2 genes (Zhang et al., 2000). The upstream regions of the maize fie genes are completely different, which is consistent with their distinct expression patterns and distinct modes of regulation. Rapid evolution of regulatory sequences may play a predominant role in plant evolution (Doebley and Lukens, 1998). This process usually leads to the functional specialization of duplicated genes.
To understand the evolutionary relationship between the two maize FIE proteins and their relationship to other plant FIE proteins, we performed a phylogenetic analysis of FIE proteins from 11 plant species. The results demonstrate that sorghum FIE and maize FIE2 are more related to each other than to maize FIE1 (Figure 7). This may be explained by the allotetraploid nature of maize, in which the donor genome that contained the fie2 gene was more closely related to the sorghum genome. This argument is consistent with the hypothesis of Gaut and Doebley (1997) that one of the two ancestral maize genomes shares a more recent common ancestor with sorghum.
fie Genes Are Expressed Differentially
Duplicated genes often have very different evolutionary fates. Redundant function is evolutionarily unstable; duplicated genes either diverge functionally or one of the versions may be lost (Theißen, 2002). The differential expression pattern of the maize fie genes suggests that they have nonredundant functions that became specialized during maize evolution.
As has been shown previously, fie1 expression is limited to developing kernels, whereas fie2 is expressed throughout development in various plant tissues (Springer et al., 2002). The spatial and temporal patterns of fie expression in developing kernels have been studied by several methods and have revealed significant differences between the transcriptional activities of the two genes. fie1 RNA shows an inducible pattern of expression in kernels with a maximum accumulation at ∼9 DAP, whereas fie2 RNA is detected at a relatively constant, low level in kernels at all stages examined. Moreover, the fie genes show different levels of expression in the embryo and endosperm. fie1 is active exclusively in the endosperm, as was shown by MPSS RNA profiling and RT-PCR of RNA isolated from the embryo and endosperm. Unlike fie1, fie2 transcripts are detected by RT-PCR and in situ hybridization predominantly in the embryo and at lower levels in the endosperm. In situ hybridization is not sensitive enough to detect fie2 transcripts in the endosperm at 15 DAP, whereas the embryo-specific expression is seen very clearly within the embryonic axis, vegetative apex, leaves, coleoptiles, and radicle but not in the scutellum.
As putative repressors of endosperm development before fertilization, fie genes should be expressed in ovules. However, fie1 mRNA is not detected in ovules by RNA gel blot analysis or by MPSS profiling (Figure 2). The high sensitivity of MPSS provides strong evidence for the absence of expression of fie1 in ovules before pollination. On the other hand, fie2 RNA is detected in ovules by RNA gel blot analysis and localized by in situ hybridization within the embryo sac (Figure 3). Of the two maize FIE proteins, only FIE2 is a plausible candidate for a repressor of endosperm development before fertilization, the function performed by the Arabidopsis FIE protein. Of course, loss-of-function mutants are required to substantiate this hypothesis.
The Arabidopsis FIE gene is expressed in both reproductive and vegetative tissues (Ohad et al., 1999; Luo et al., 2000). Loss-of-functions alleles of Arabidopsis FIE produce pleiotropic phenotypes, including initiation of endosperm development without fertilization, embryo abortion at early stages, premature flowering by seedling shoots, and formation of flower-like structures along the roots and hypocotyls (Ohad et al., 1996, 1999; Kinoshita et al., 2001). These results suggest that the FIE protein encoded by a single-copy gene in the Arabidopsis genome may form distinct complexes in different plant tissues and participate in the regulation of several developmental programs. We hypothesize that during evolution, fie2 performed a function orthologous to that of Arabidopsis FIE but the duplicated fie1 gene diverged functionally, acquiring a specialized role likely related to endosperm development. The conservation of protein structure suggests that both maize FIE proteins function as negative regulators of transcription by means of chromatin-mediated gene silencing. However, because of their distinct spatial and temporal patterns of expression, they might regulate different pathways.
fie Genes Show Different Parent-of-Origin Effects
A prominent feature of the Arabidopsis FIS genes is their parent-of-origin effect on seed development. The wild-type paternal alleles do not rescue maternally derived fis mutant alleles. This effect was explained by partial (FIE and MEDEA) or complete (FIS2) silencing of paternally derived alleles (Ohad et al., 1996; Grossniklaus et al., 1998; Kinoshita et al., 1999; Vielle-Calzada et al., 1999; Luo et al., 2000; Yadegari et al., 2000).
To investigate a parent-of-origin effect on maize fie gene expression, we conducted several experiments to monitor the accumulation of the paternal and maternal fie RNAs in developing kernels. The fie1 paternal allele shows imprinting with no expression of the paternal allele detected at any developmental stage tested (Figure 4). This is similar to findings in the Arabidopsis FIS2 gene (Luo et al., 2000). Although FIE1 and FIS2 are different types of proteins, their genes have in common endosperm-specific expression. A FIS2–β-glucuronidase fusion construct shows activity only in the endosperm during all stages of seed development (Luo et al., 2000). fie1 also is expressed only in the endosperm. This finding is consistent with observations that imprinted genes are expressed primarily in the endosperm rather than in the embryo (Haig and Westoby, 1989; Alleman and Doctor, 2000).
Conversely, the paternal fie2 allele is silenced only early in kernel development, with expression after 5 DAP. The delayed expression of paternal alleles is characteristic of the Arabidopsis MEDEA and FIE genes (Kinoshita et al., 1999; Vielle-Calzada et al., 1999; Luo et al., 2000). Genes that are expressed in both embryo and endosperm, such as FIE, MEDEA, and fie2, show only partial silencing of the paternal alleles early in development (Ohad et al., 1996; Chaudhury et al., 1998; Grossniklaus et al., 1998, 2001; Kinoshita et al., 1999). Later in development, silencing breaks down and both alleles are expressed equally in the vegetative tissues of mature plants. Alternatively, the delayed expression of paternal alleles may be explained by delayed activation of the entire paternal genome after fertilization (Vielle-Calzada and Grossniklaus, 2000). If this is the case, this trend should apply to any gene transmitted paternally. To discriminate between these alternative hypotheses, the parent-of-origin expression of many genes will need to be studied.
The expression pattern of fie2 parallels the expression pattern of Arabidopsis FIE. Given the similarities in protein sequence, expression pattern, and mode of imprinting, we speculate that fie2 is the functional ortholog of the Arabidopsis FIE gene.
fie Genes Agree with the Two-Island Rule
It has been proposed that in plants, the presence of repeated sequences is a common feature of epigenetically silenced and imprinted genes (Alleman and Doctor, 2000). We have analyzed the genomic sequences of both genes for direct and inverted repeats and have found a complex repetitive structure upstream of fie2 but none near fie1. A 2.6-kb symmetrical cluster is adjacent to the putative fie2 promoter, and another 1-kb cluster is located farther upstream (Figure 1B). The entire 6-kb upstream fragment shares no obvious similarity to any known transposable or repetitive DNA. These upstream repeats might constitute a cis-acting element that controls the transcription of fie2. Hypothetical proteins might be involved in binding to these repeats to activate or repress gene expression. These complexes might temporarily associate with the upstream sequence early but dissociate later during kernel development, allowing activation of the paternally transmitted allele.
The genomic sequence of the fie1 locus shows no repetitive structures. A special feature of the fie1 gene is a 290-bp intron positioned in the 5′ UTR. There are many examples of maize genes with introns in the 5′ UTR (Shaw et al., 1994) but no indications of their involvement in genomic imprinting. The first exon and intron very often are required for the high-level expression of transgenes that may be the result of the increased level or stability of the mature cytoplasmic mRNA (Kim and Guiltinan, 1999). It is likely that the 5′ UTR intron of fie1 plays a role in mRNA stability or in determining tissue-specific expression.
As a way to find “imprint” marks, we analyzed the distribution of CpG islands in the genomic sequences of the fie genes. It was proposed that CpG islands might be common elements in mammalian imprinted genes (Wutz et al., 1997). Comparative analysis of the mouse and human 1-Mb imprinted domain revealed a two-island rule for imprinted genes (Onyango et al., 2000). Imprinted genes show two or more conserved CpG islands upstream or within the gene, whereas nonimprinted genes have at most one CpG island. CpG islands normally are unmethylated and associated with actively transcribed genes (Ponger et al., 2001), but allele-specific methylation of CpG islands marks genes for silencing in mammals (Wutz et al., 1997). Computation of CpG islands within fie1 and fie2 genomic sequences revealed two CpG islands in fie1 and one CpG island in fie2 (Figure 6). This observation is in remarkable agreement with the two-island rule for mammals proposed by Onyango et al. (2000). The fie1 gene, in which the paternal allele is silenced permanently during all stages of kernel development, contains two CpG islands. fie2, in which the paternal allele is only delayed in expression, contains one CpG island. We speculate that CpG islands might be imprint marks for fie1.
METHODS
Gene-Specific Probes
To discriminate between maize (Zea mays) fie1 and fie2 during hybridization experiments, gene-specific probes were made by PCR of the cDNA 3′ untranslated regions (UTRs). Primers 5′-CTGCTTCCA-GCTCCAAAC-3′ and 5′-TTATTCATCTCATCCACGGTG-3′ amplify a 287-bp fragment from nucleotides 1466 to 1753 of the fie1 cDNA. Primers 5′-ATCCGAGCTCCAGAAACTGA-3′ and 5′-ATGATTTAA-CGTTATCTGTTACCCA-3′ amplify a 270-bp fragment from nucleotides 1320 to 1590 of the B73 fie2 cDNA.
Cloning and Sequencing of fie Genomic Fragments
The Mo17 BAC genomic library was screened with full-length fie1 and fie2 cDNAs. Five BAC clones for each gene were identified and confirmed by DNA gel blot hybridization with gene-specific probes. HindIII and EcoRI BAC fragments were subcloned into pBluescript II KS+ (Stratagene) and hybridized with fie-specific probes, and positive clones were submitted for sequencing.
DNA Sequence Analysis
DNA assembly was performed using the Sequencher program (Gene Code Corp., Ann Arbor, MI). Sequence and dot plot analyses were performed with GCG programs (Genetics Computer Group, Madison, WI). The CpG islands were computed using the newcpgreport program, which is part of the EMBOSS suite (ftp://ftp.ebi.ac.uk/pub/databases/cpgisle/).
Phylogenetic Analysis
Putative plant FIE proteins were deduced from ESTs identified by TBLASTX (Altschul et al., 1997) in GenBank (Table 1). Animal WD Polycomb proteins included in the analysis are shown in Table 1. Three GBB (Guanine Nucleotide Binding Protein β-Subunit 2) WD-motif proteins (Sondek et al., 1996) were used as an outgroup in the analysis. Protein sequences were aligned using the CLUSTAL W program (Thompson et al., 1994). Phylogenetic analysis was performed using parsimony and neighbor-joining methods implemented with the PAUP program (Swofford, 1998). Bootstrap (Felsenstein, 1985) and jackknife (Wu, 1986) resampling analyses with 500 replicates were performed to assess the degree of support for each branch on the phylogenetic tree. Maximum-likelihood analysis also was performed using the proMLK algorithm of the PHYLIP package (Phylogeny Inference Package, version 3.6a2.1; http://evolution.genetics.washington.edu/phylip.html) by J. Felsenstein.
RNA Gel Blot Analysis
Total RNA was extracted from 1 g of material using a hot-phenol extraction procedure and selective precipitation with 4 M LiCl to remove traces of DNA and small RNA species (Verwoerd et al., 1989; Brugière et al., 1999). For each time point, kernels were collected from two ears harvested from two different plants (replications) from either the B73 or the Mo17 inbred line. RNA was quantified using a spectrophotometer (Beckman Instruments, Fullerton, CA) at 260 nm. Poly(A) was prepared from total RNA (400 μg) using the Oligotex poly(A) purification kit (Qiagen, Valencia, CA). Electrophoretic separation was performed on 1.5% agarose gels containing 5% (v/v) of a solution of 37% formaldehyde in 3-(N-morpholino)-propanesulfonic acid buffer [0.02 M 3-(N-morpholino)-propanesulfonic acid, pH 7.0, 5 mM sodium acetate, and 1 mM EDTA]. Gels were blotted to nylon membranes (Roche Molecular Biochemicals, Mannheim, Germany) using the TurboBlotter (Schleicher & Schuell, Keene, NH), with 20 × SSC (1 × SSC is 150 mM NaCl and 15 mM sodium citrate) as the transfer buffer. Blots were probed with 32P-labeled PCR fragments of fie1 or fie2 cut from the 3′ UTRs of the appropriate EST clones. Probe sequences share no homology, which avoids cross-hybridization.
Distinguishing fie mRNAs in Reciprocal Crosses
Reciprocal crosses between B73 and Mo17 inbred lines were made, and F1 kernels were sampled at 2, 5, 10, and 15 days after pollination. Total RNA was isolated using the Purescript RNA Isolation Kit (catalog number R-5000A) from Gentra Systems (Minneapolis, MN). cDNA synthesis was performed with the Superscript First-Strand Synthesis System (catalog number 11904-018) from Invitrogen. PCR was performed with Pwo DNA polymerase (catalog number 1,644,947) from Roche Molecular Biochemicals. Primers used to amplify fie1 cDNA were 5′-AGGCGAGATCTATGTCTGGGAAGTGCAGTC-3′ and 5′-CAACCAGCACGGAGTACGATCGATGTGAA-3′. The product was 300 bp, and the B73 allele differed from the Mo17 allele by a 12-bp deletion. Primers designed to amplify fie2 were based on the MITE insertion in the B73 allele. The forward primer (5′-CGTGAAGGCAAAATCTACGTGTGG-3′), positioned in the 11th exon, is common to both genotypes. In B73, fie2 poly(A) transcripts terminate in the middle of the MITE insertion that became part of the 3′ UTR. The B73-specific reverse primer is 5′-CATTACGTTACAAATATGTGAACCAAACG-3′ and is designed for the MITE sequence. The Mo17-specific reverse primer is 5′-CAGAACAAACAGATG-ACAACGGTTCCCAAAG-3′ and is designed for the 3′ UTR of cDNA that has no homology with MITE sequences. PCR fragments were separated on 1% agarose gels. This primer combination allows the monitoring of parental allele expression in reciprocal crosses. Primers for the biallelic expressed gene were 5′-GGGACGAAG-ATAAAACG-3′ and 5′-GCCAAACAACATTTTGTATAT-3′. α-Tubulin primers were 5′-AGCCCGATGGCACCATGCCCAGTGATACCT-3′ and 5′-AACACCAAGAATCCCTGCAGCCCAGTGC-3′.
In Situ Hybridization
In situ hybridization was performed using the protocol of Jackson (1991) modified according to Bradley et al. (1993). Sense and antisense mRNA probes of 300 bp corresponding to the 3′ UTR of the fie2 gene were labeled nonisotopically with digoxigenin-UTP by in vitro transcription with T7 and T3 RNA polymerases (Roche Molecular Biochemicals). Probes were hybridized to fixed sections of maize tissue from ovules at silking and kernels at 5, 8, and 12 days after pollination. After extensive washing to remove unbound probe, a signal was detected with anti-digoxigenin antibodies conjugated with alkaline phosphatase to mediate the color reaction (Roche Molecular Biochemicals). fie2 mRNAs were detected specifically with the antisense probe. The sense probe did not hybridize; therefore, it served as a negative control.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Accession Numbers
The sequences described in this article have been deposited in GenBank under the accession numbers AY150645 (fie1 genomic locus), AY150646 (fie2 genomic locus), AY150643 (fie2 cDNA from Mo17), AY150644 (fie2 cDNA from B73), AY150282 (fie cDNA from Eucalyptus grandis), and AY150383 (fie cDNA from Catalpa speciosa). Accession numbers for the other sequences mentioned are as follows: Arabidopsis FIE gene (AB025628), bz2 locus (AF391808), MILT retroelement (AF050449), EST MEST80-E04.T3 (BM074115), and fie1 cDNA (AY061964).
Acknowledgments
The authors thank Tim Helentjaris, Norberto Bruggerie, Mei Guo, Mary Rupe, Wes Bruce, Carl Simmons, and Pedro Navarro for sharing ideas, materials, and bioinformatic assistance and Nathan Springer and Isaac Boer for critically reading the manuscript.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.006759.
References
- Alleman, M., and Doctor, J. (2000). Genomic imprinting in plants: Observation and evolutionary implications. Plant Mol. Biol. 43, 147–161. [DOI] [PubMed] [Google Scholar]
- Altschul, S.F., Madden, T.L., Schaffer, A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, D., Carpenter, R., Somer, H., Hartley, N., and Coen, E. (1993). Complementary floral homeotic phenotypes result from opposite orientations of a transposon at the plena locus of Antirrhinum. Cell 72, 85–95. [DOI] [PubMed] [Google Scholar]
- Brenner, S., et al. (2000. a). Gene expression analysis by massively parallel signature sequencing (MPSS) on microbead arrays. Nat. Biotechnol. 18, 630–634. [DOI] [PubMed] [Google Scholar]
- Brenner, S., et al. (2000. b). In vitro cloning of complex mixtures of DNA on microbeads: Physical separation of differentially expressed cDNAs. Proc. Natl. Acad. Sci. USA 97, 1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugière, N., Dubois, F., Limami, A.M., Lelandais, M., Roux, Y., Sangwan, R.S., and Hirel, B. (1999). Glutamine synthetase in the phloem plays a major role in controlling proline production. Plant Cell 10, 1995–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury, A.M., and Berger, F. (2001). Maternal control of seed development. Cell Dev. Biol. 112, 381–386. [DOI] [PubMed] [Google Scholar]
- Chaudhury, A.M., Craig, S., Dennis, E., and Peacock, W. (1998). Ovule and embryo development, apomixis and fertilization. Curr. Opin. Plant Biol. 1, 26–31. [DOI] [PubMed] [Google Scholar]
- Chaudhury, A.M., Koltunow, A., Payne, T., Luo, M., Tucker, M.R., Dennis, E.S., and Peacock, W.J. (2001). Control of early seed development. Annu. Rev. Cell Dev. Biol. 17, 677–699. [DOI] [PubMed] [Google Scholar]
- Chaudhury, A.M., Ming, L., Miller, C., Craig, S., Dennis, E.S., and Peacock, W.J. (1997). Fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 94, 4223–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisenko, O.N., and Bomsztyk, K. (1997). The product of the murine homolog of the Drosophila extra sex combs gene displays transcriptional repressor activity. Mol. Cell. Biol. 17, 4707–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley, J., and Lukens, L. (1998). Transcriptional regulators and the evolution of plant form. Plant Cell 10, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39, 783–791. [DOI] [PubMed] [Google Scholar]
- Fu, H., Park, W., Yan, X., Zheng, Z., Shen, B., and Dooner, H.K. (2001). The highly recombinogenic bz locus lies in an unusually gene-rich region of the maize genome. Proc. Natl. Acad. Sci. USA 15, 8903–8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner-Garden, M., and Frommer, M. (1987). CpG islands in vertebrate genomes. J. Mol. Biol. 196, 261–282. [DOI] [PubMed] [Google Scholar]
- Gaut, B.S., and Doebley, J.F. (1997). DNA sequence evidence for the segmental allotetraploid origin of maize. Proc. Natl. Acad. Sci. USA 94, 6809–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimanelli, D., Leblanc, O., Perotti, E., and Grossniklaus, U. (2001). Developmental genetics of gametophytic apomixis. Trends Genet. 17, 597–604. [DOI] [PubMed] [Google Scholar]
- Grossniklaus, U., Spillane, C., Page, D.R., and Kohler, C. (2001). Genomic imprinting and seed development: Endosperm formation with and without sex. Curr. Opin. Plant Biol. 4, 21–27. [DOI] [PubMed] [Google Scholar]
- Grossniklaus, U., Vielle-Calzada, J.P., Hoeppner, M.A., and Gagliano, W.B. (1998). Maternal control of embryogenesis by MEDEA, a Polycomb group gene in Arabidopsis. Science 280, 446–450. [DOI] [PubMed] [Google Scholar]
- Haig, D., and Westoby, M. (1989). Parent specific gene expression and the triploid endosperm. Am. Nat. 123, 147–155. [Google Scholar]
- Helentjaris, T. (1995). Atlas of duplicated sequences. Maize Newsl. 69, 67–81. [Google Scholar]
- Jackson, D.P. (1991). In situ hybridization in plants. In Molecular Plant Pathology: A Practical Approach, D.J. Bowles, S.J. Gurr, and M. McPherson, eds (Oxford, UK: Oxford University Press), pp. 63–74.
- Kim, K.N., and Guiltinan, M.J. (1999). Identification of cis-acting elements important for expression of the starch-branching enzyme I gene in maize endosperm. Plant Physiol. 121, 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, T., Harada, J.J., Goldberg, R.B., and Fischer, R.L. (2001). Polycomb repression of flowering during early plant development. Proc. Natl. Acad. Sci. USA 98, 14156–14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, T., Yadegari, R., Harada, J.J., Goldberg, R.B., and Fischer, R.L. (1999). Imprinting of the MEDEA Polycomb gene in the Arabidopsis endosperm. Plant Cell 11, 1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow, A. (2001). Apomixis takes center stage. Trends Plant Sci. 6, 543. [Google Scholar]
- Korf, I., Fan, Y., and Strome, S. (1998). The Polycomb group in Caenorhabditis elegans and maternal control of germline development. Development 125, 2469–2478. [DOI] [PubMed] [Google Scholar]
- Luo, M., Bilodeau, P., Dennis, E.S., Peacock, W.J., and Chaudhury, A. (2000). Expression and parent-of-origin effects for FIS2, MEA and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc. Natl. Acad. Sci. USA 97, 10637–10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, M., Bilodeau, P., Koltunow, A., Dennis, E.S., Peacock, W.J., and Chaudhury, A.M. (1999). Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 5, 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, J., Li, R., Morgan, K., and Simon, J. (1997). Revolutionary conservation and predicted structure of the Drosophila extra sex combs repressor protein. Mol. Cell. Biol. 17, 6663–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad, N., Margossian, L., Hus, Y., Williams, C., Repetti, P., and Fischer, R.L. (1996). A mutation that allows endosperm development without fertilization. Proc. Natl. Acad. Sci. USA 93, 5319–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad, N., Yadegari, R., Margossian, L., Hannon, M., Michaeli, D., Harada, J.J., Goldberg, R.B., and Fischer, R.L. (1999). Mutations in FIE, a WD Polycomb group gene, allow endosperm development without fertilization. Plant Cell 11, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango, P., Miller, W., Lehoczky, J., Leung, C.T., Birren, B., Wheelan, S., Dewar, K., and Feinberg, A.P. (2000). Sequence and comparative analysis of the mouse 1-megabase region orthologous to the human 11p15 imprinted domain. Genome Res. 10, 1697–1710. [DOI] [PubMed] [Google Scholar]
- Pirrotta, V. (1998). Polycombing the genome: PcG, trxG, and chromatin silencing. Cell 93, 333–336. [DOI] [PubMed] [Google Scholar]
- Ponger, L., Duret, L., and Mouchiroud, D. (2001). Determinants of CpG islands: Expression in early embryo and isochore structure. Genome Res. 11, 1854–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik, W., and Walter, J. (2001). Genomic imprinting: Parental influence on the genome. Nat. Rev. Genet. 2, 21–32. [DOI] [PubMed] [Google Scholar]
- Reiser, L., and Fischer, R. (1993). The ovule and the embryo sac. Plant Cell 5, 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietzler, M., Bittner, M., Kolanus, W., Schuster, A., and Holzmann, B. (1998). The human WD repeat protein WAIT-1 specifically interacts with the cytoplasmic tails of beta7-integrins. J. Biol. Chem. 273, 27459–27466. [DOI] [PubMed] [Google Scholar]
- Shaw, J.R., Ferl, R.J., Baier, J., St. Clair, D., Carson, C., McCarty, D.R., and Hannah, L.C. (1994). Structural features of the maize sus1 gene and protein. Plant Physiol. 106, 1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondek, J., Bohm, A., Lambright, D.G., Hamm, H.E., and Sigler, P.B. (1996). Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature 379, 369–374. [DOI] [PubMed] [Google Scholar]
- Sorensen, M.B., Chaudhury, A.M., Robert, H., Bancharel, E., and Berger, F. (2001). Polycomb group genes control pattern formation in plant seed. Curr. Biol. 11, 277–281. [DOI] [PubMed] [Google Scholar]
- Spillane, C., MacDougall, C., Stock, C., Kohler, C., Vielle-Calzada, J.P., Nunes, S.M., Grossniklaus, U., and Goodrich, J. (2000). Interaction of the Arabidopsis Polycomb group proteins FIE and MEA mediates their common phenotypes. Curr. Biol. 10, 1535–1538. [DOI] [PubMed] [Google Scholar]
- Springer, N.M., Danilevskaya, O.N., Hermon, P., Helentjaris, T.G., Phillips, R.L., Kaeppler, H.F., and Kaeppler, S.M. (2002). Sequence relationships, conserved domains, and expression patterns for maize homologs of the Polycomb group genes E(z), esc, and E(Pc). Plant Physiol. 128, 1332–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford, D.L. (1998). PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods), Version 4. (Sunderland, MA: Sinauer Associates).
- Theißen, G. (2002). Secret life of genes. Nature 415, 741. [DOI] [PubMed] [Google Scholar]
- Thompson, J.D., Higgins, D.G., and Gibson, T.J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsen, J.L., Duran, K.L., and Bartolomei, M.S. (1998). Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 12, 3693–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie, F., Furuyama, T., Prasad-Sinha, J., Jane, E., and Harte, P.J. (2001). The Drosophila Polycomb group proteins ESC and E(Z) are present in a complex containing the histone-binding protein p55 and the histone deacetylase RPD3. Development 123, 275–286. [DOI] [PubMed] [Google Scholar]
- van der Vlag, J., and Otte, A.P. (1999). Transcriptional repression mediated by the human Polycomb-group protein EED involves histone deacetylation. Nat. Genet. 23, 474–478. [DOI] [PubMed] [Google Scholar]
- Verwoerd, T.C., Dekker, B.N.M., and Hoekema, A. (1989). A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 17, 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielle-Calzada, B.R., and Grossniklaus, U. (2000). Delayed activation of the paternal genome during seed development. Nature 404, 91–94. [DOI] [PubMed] [Google Scholar]
- Vielle-Calzada, J.P., Thomas, J., Spillane, C., Coluccio, A., Hoeppner, M.A., and Grossniklaus, U. (1999). Maintenance of genomic imprinting at the Arabidopsis medea locus requires zygotic DDM1 activity. Genes Dev. 13, 2971–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkenoog, R., Spielman, M., Adams, S., Fischer, R.L., Dickinson, H.G., and Scott, R.J. (2000). Hypomethylation promotes autonomous endosperm development and rescues postfertilization lethality in fie mutants. Plant Cell 12, 2271–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkenoog, R.S., and Scott, R. (2001). Autonomous endosperm development in flowering plants: How to overcome the imprinting problem? Sex. Plant Reprod. 14, 184–189. [DOI] [PubMed] [Google Scholar]
- Wessler, S.R. (2001). Plant transposable elements: A hard act to follow. Plant Physiol. 125, 149–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C.F.J. (1986). Jackknife, bootstrap and other resampling methods in regression analysis (with discussion). Ann. Stat. 14, 1261–1350. [Google Scholar]
- Wutz, A., Smrzka, O.W., Schweifer, N., Schellander, K., Wagner, E.F., and Barlow, D.P. (1997). Imprinted expression of the Igf2r gene depends on an intronic CpG island. Nature 389, 745–749. [DOI] [PubMed] [Google Scholar]
- Yadegari, R., Kinoshita, T., Lotan, O., Cohen, G., Katz, A., Nakashima, K., Harada, J.J., Goldberg, R.B., Fischer, R.L., and Ohad, N. (2000). Mutations in the FIE and MEA genes that encode interacting Polycomb proteins cause parent-of-origin effects on seed development by distinct mechanisms. Plant Cell 12, 2367–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., Chopra, S., and Peterson, T. (2000). A segmental gene duplication generated differentially expressed myb homologous genes in maize. Plant Cell 12, 2311–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]