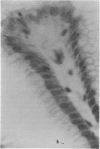
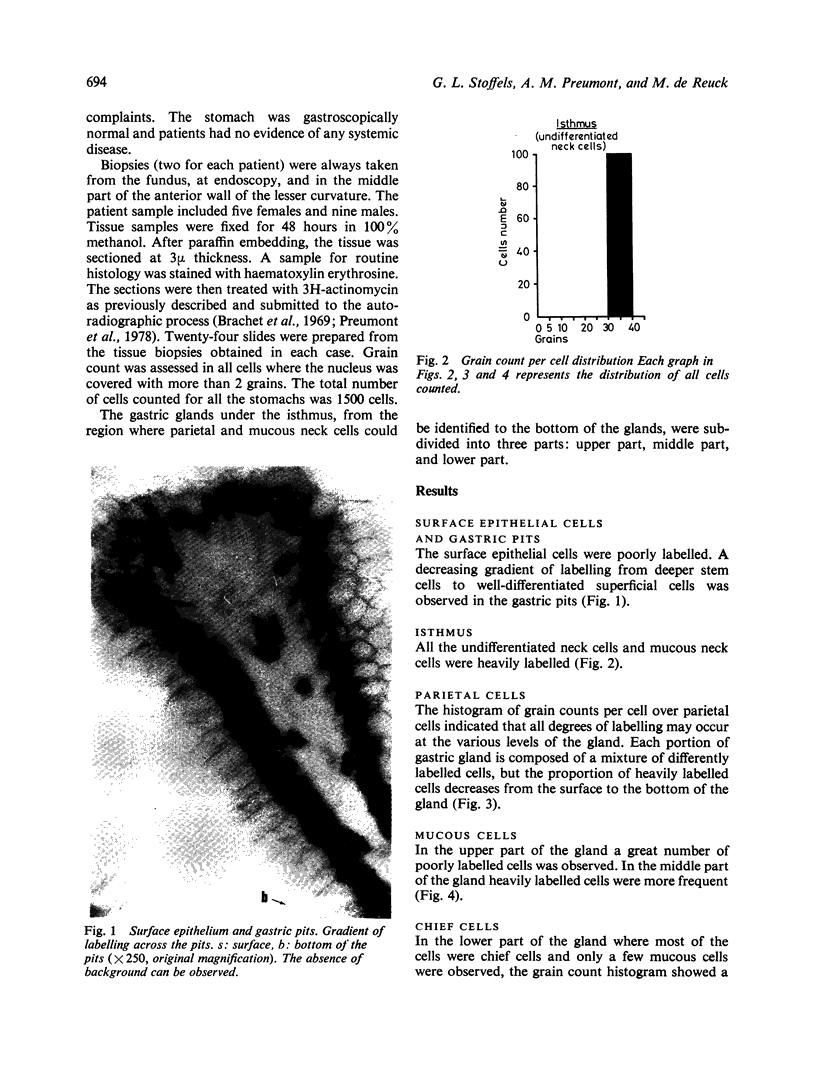
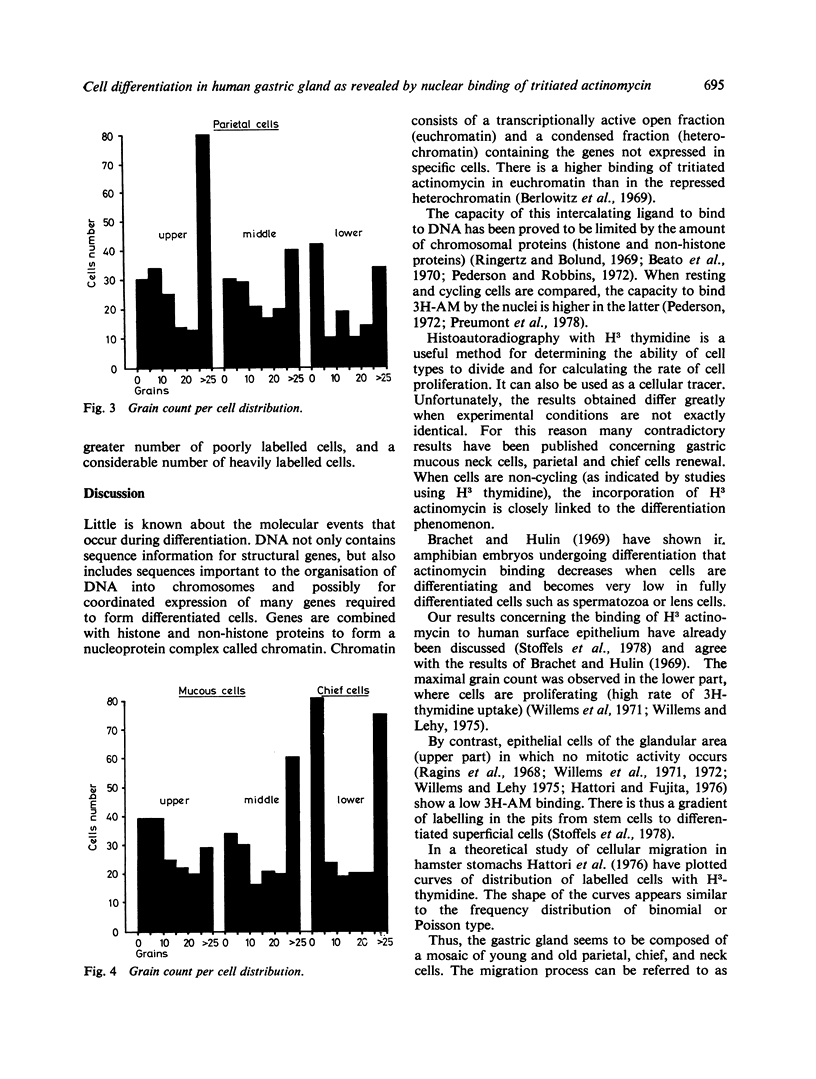
Abstract
The nuclear binding of H3 actinomycin, which is closely linked to the differentiation phenomenon, was studied in human normal gastric mucosa. Actinomycin binding decreases in cells which differentiate and becomes very low in fully differentiated cells. In the gastric pits, there is a decreasing gradient of labelling from the deeper stem cells to the well-differentiated superficial cells. This indicates that migration and renewal of the surface epithelium occurs following a 'pipe-line' system. All the undifferentiated stem cells are labelled. Where the parietal cells are concerned all degrees of labelling are observed at various levels with a decreasing proportion of labelling from the surface to the bottom of the gland. With mucous cells and chief cells in the upper part of the gland a great number of poorly labelled mucous neck cells is observed. In the middle and lower part of the gland there is a new growth of heavily labelled cells. This means that in the normal human stomach chief cells probably do not originate from mucous cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M., Seifart K. H., Sekeris C. E. The effect of cortisol on the binding of actinomycin D to and on the template activity of isolated rat liver chromatin. Arch Biochem Biophys. 1970 May;138(1):272–284. doi: 10.1016/0003-9861(70)90308-5. [DOI] [PubMed] [Google Scholar]
- Berlowitz L., Pallotta D., Sibley C. H. Chromatin and histones: binding of tritiated actinomycin D to heterochromatin in mealy bugs. Science. 1969 Jun 27;164(3887):1527–1529. doi: 10.1126/science.164.3887.1527. [DOI] [PubMed] [Google Scholar]
- Brachet J., Hulin N. Binding of tritiated actinomycin and cell differentiation. Nature. 1969 May 3;222(5192):481–482. doi: 10.1038/222481a0. [DOI] [PubMed] [Google Scholar]
- CREAMER B., SHORTER R. G., BAMFORTH J. The turnover and shedding of epithelial cells. I. The turnover in the gastro-intestinal tract. Gut. 1961 Jun;2:110–118. doi: 10.1136/gut.2.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean G. P. Effect of hypophysectomy on the gastric mucosa of the rat. Gut. 1968 Jun;9(3):332–342. doi: 10.1136/gut.9.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean G. P., Gunn A. A., Rumsey R. D. The effects of vagotomy on the gastric mucosa of the rat. Scand J Gastroenterol. 1969;4(8):675–680. doi: 10.3109/00365526909180653. [DOI] [PubMed] [Google Scholar]
- Crean G. P., Rumsey R. D. Hyperplasia of the gastric mucosa during pregnancy and lactation in the rat. J Physiol. 1971 May;215(1):181–197. [PMC free article] [PubMed] [Google Scholar]
- HUNT T. E., HUNT E. A. Radioautographic study of proliferation in the stomach of the rat using thymidine-H3 and compound 48/80. Anat Rec. 1962 Apr;142:505–517. doi: 10.1002/ar.1091420408. [DOI] [PubMed] [Google Scholar]
- Hattori T., Fujita S. Tritiated thymidine autoradiographic study on cellular migration in the gastric gland of the golden hamster. Cell Tissue Res. 1976 Sep 14;172(2):171–184. doi: 10.1007/BF00226025. [DOI] [PubMed] [Google Scholar]
- Helander H. F. Ultrastructure and function of gastric parietal cells in the rat during development. Gastroenterology. 1969 Jan;56(1):35–52. [PubMed] [Google Scholar]
- LIPKIN M., SHERLOCK P., BELL B. CELL PROLIFERATION KINETICS IN THE GASTROINTESTINAL TRACT OF MAN. II. CELL RENEWAL IN STOMACH, ILEUM, COLON, AND RECTUM. Gastroenterology. 1963 Dec;45:721–729. [PubMed] [Google Scholar]
- MACDONALD W. C., TRIER J. S., EVERETT N. B. CELL PROLIFERATION AND MIGRATION IN THE STOMACH, DUODENUM, AND RECTUM OF MAN: RADIOAUTOGRAPHIC STUDIES. Gastroenterology. 1964 Apr;46:405–417. [PubMed] [Google Scholar]
- MESSIER B., LEBLOND C. P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat. 1960 May;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- Matsuyama M., Suzuki H. Differentiation of immature mucous cells into parietal, argyrophil, and chief cells in stomach grafts. Science. 1970 Jul 24;169(3943):385–387. doi: 10.1126/science.169.3943.385. [DOI] [PubMed] [Google Scholar]
- Pederson T., Robbins E. Chromatin structure and the cell division cycle. Actinomycin binding in synchronized HeLa cells. J Cell Biol. 1972 Nov;55(2):322–327. doi: 10.1083/jcb.55.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preumont A. M., Van Gansen P., Brachet J. Cytochemical study of human lymphocytes stimulated by PHA in function of donor age. Mech Ageing Dev. 1978 Jan;7(1):25–32. doi: 10.1016/0047-6374(78)90050-7. [DOI] [PubMed] [Google Scholar]
- Ringertz N. R., Bolund L. Actinomycin binding capacity of deoxyribonucleoprotein. Biochim Biophys Acta. 1969 Jan 21;174(1):147–154. doi: 10.1016/0005-2787(69)90237-8. [DOI] [PubMed] [Google Scholar]
- Seelig L. L., Jr, Winborn W. B., Weser E. Changes in gastric glandular cell kinetics after small bowel resection in the rat. Gastroenterology. 1978 Jan;74(1):1–6. [PubMed] [Google Scholar]
- Stoffels G. L., Preumont A. M., de Reuck M. Nuclear binding of tritiated actinomycin in surface epithelial cells from normal stomach and atrophic gastritis. Gut. 1978 Oct;19(10):870–874. doi: 10.1136/gut.19.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems G., Galand P., Vansteenkiste Y., Zeitoun P. Cell population kinetics of zymogen and parietal cells in the stomach of mice. Z Zellforsch Mikrosk Anat. 1972;134(4):505–518. doi: 10.1007/BF00307670. [DOI] [PubMed] [Google Scholar]
- Willems G., Lehy T. Radioautographic and quantitative studies on parietal and peptic cell kinetics in the mouse. A selective effect of gastrin on parietal cell proliferation. Gastroenterology. 1975 Aug;69(2):416–426. [PubMed] [Google Scholar]
- Willems G., Vansteenkiste Y., Verbeustel S. Autoradiographic study of cell renewal in fundic mucosa of fasting dogs. Acta Anat (Basel) 1971;80(1):23–32. doi: 10.1159/000143671. [DOI] [PubMed] [Google Scholar]