Abstract
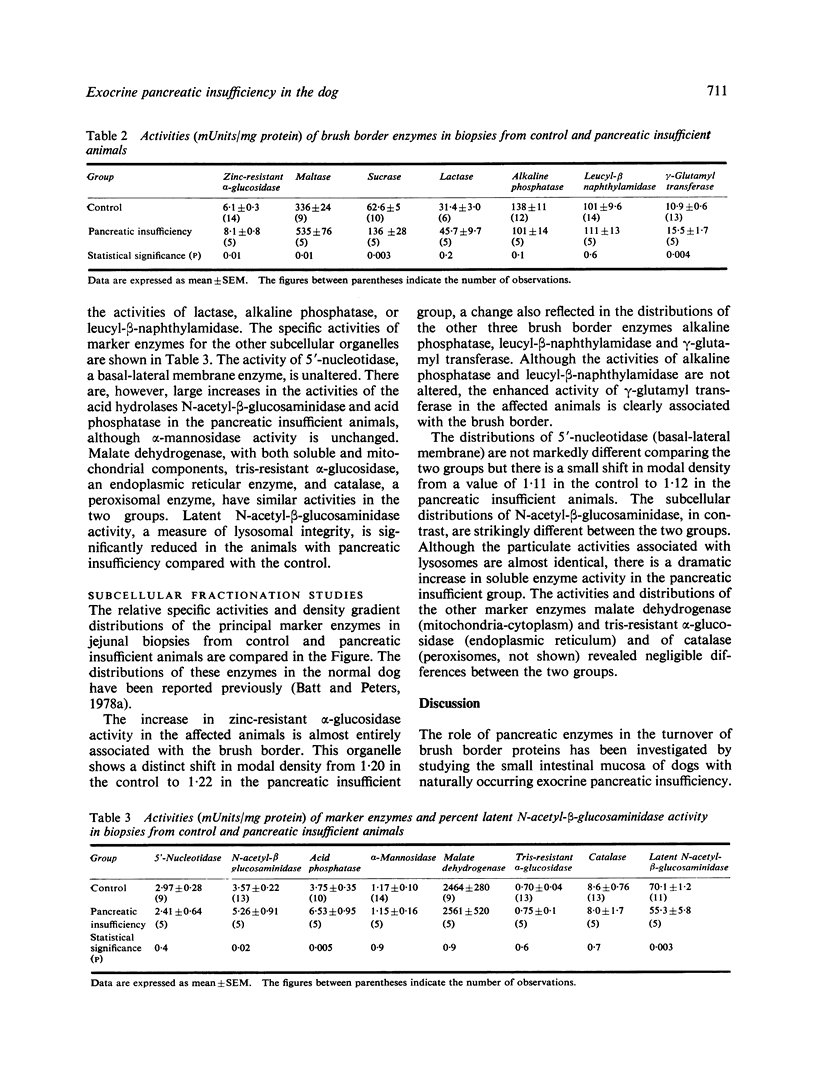
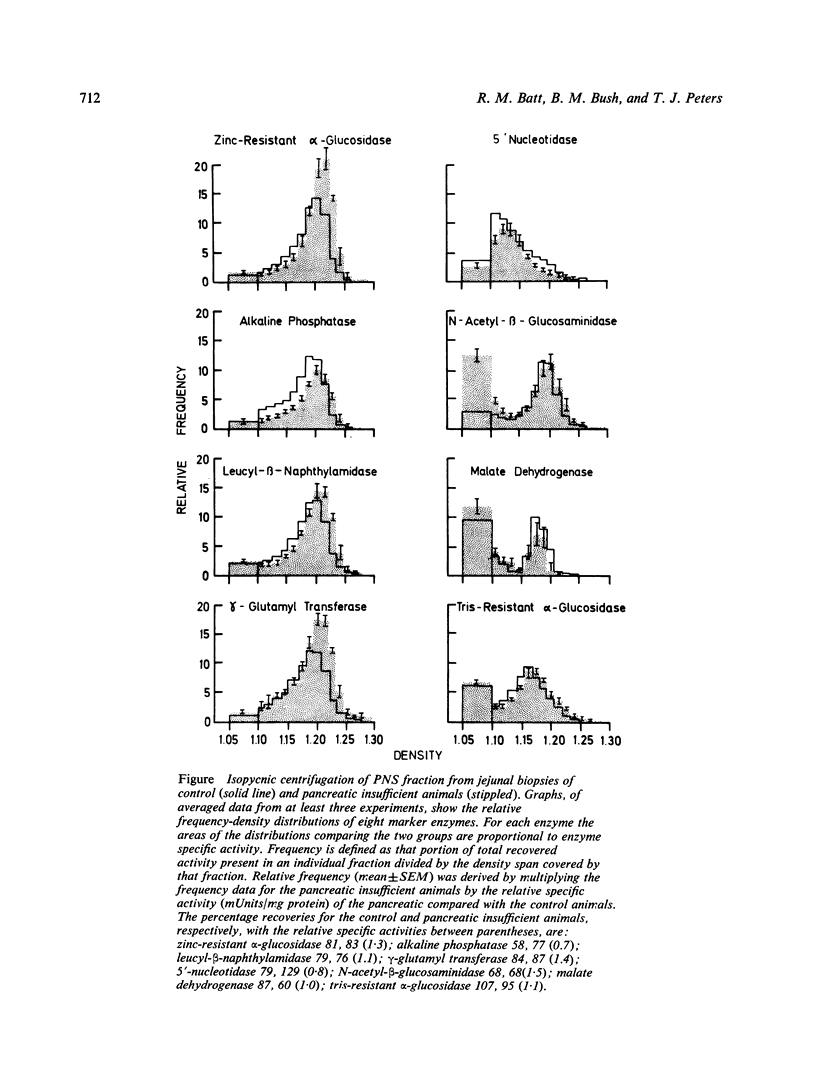
The roles of extracellular and intracellular mechanisms in the degradation of brush border proteins have been investigated by studying the small intestinal mucosa of dogs with naturally occurring exocrine pancreatic insufficiency. Peroral jejunal biopsies were homogenised and the organelles separated by isopycnic centrifugation on continuous sucrose density gradients. The distributions of marker enzymes for the principal subcellular organelles were determined in the gradients and related to the specific activities in the homogenates. There were increased activities of the brush border carbohydrases zinc-resistant α-glucosidase, maltase and sucrase in the pancreatic insufficient animals, but no change in lactase activity. The activity of γ-glutamyl transferase was also higher in the affected group; the activities of two other brush border enzymes, alkaline phosphatase and leucyl-β-naphthylamidase, however, were unaltered. These findings with an increase in the modal density of the brush border from 1·20 to 1·22 are consistent with an enhanced glycoprotein content of the microvillus membrane. There were also rises in the activities of lysosomal enzymes. N-Acetyl-β-glucosaminidase activity was increased in the soluble fractions and the percentage latent enzyme activity was reduced, findings indicative of an increased fragility of the lysosomal membrane. There were no marked alterations in the activities or density gradient distributions of marker enzymes for the other organelles, stressing the specificity of the changes in the brush borders and lysosomes. These findings are compatible with the degradation of certain exposed brush border proteins by pancreatic proteases and suggest that when this is defective, intracellular degradative mechanisms may be stimulated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Kinzie J. L. Regulation of small intestinal protein metabolism. Gastroenterology. 1973 Mar;64(3):471–496. [PubMed] [Google Scholar]
- Alpers D. H., Tedesco F. J. The possible role of pancreatic proteases in the turnover of intestinal brush border proteins. Biochim Biophys Acta. 1975 Aug 5;401(1):28–40. doi: 10.1016/0005-2736(75)90338-7. [DOI] [PubMed] [Google Scholar]
- Arvanitakis C., Olsen W. A. Intestinal mucosal disaccharidases in chronic pancreatitis. Am J Dig Dis. 1974 May;19(5):417–421. doi: 10.1007/BF01255605. [DOI] [PubMed] [Google Scholar]
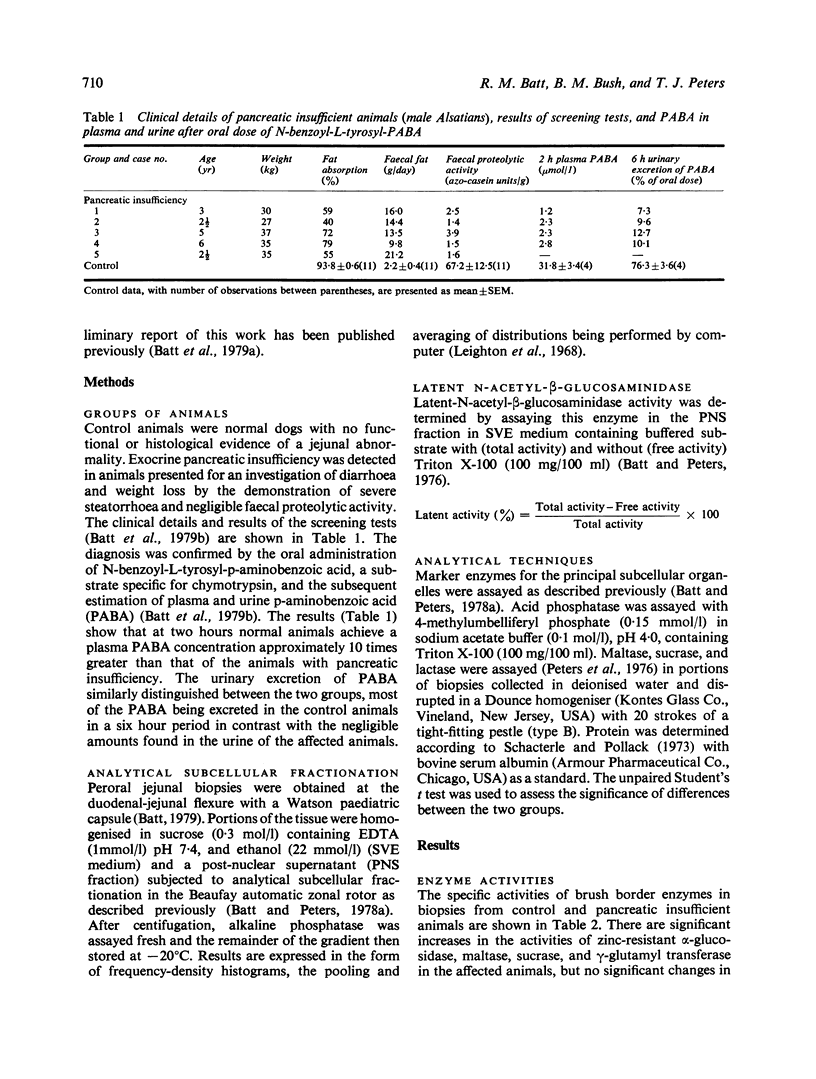
- Batt R. M., Bush B. M., Peters T. J. A new test for the diagnosis of exocrine pancreatic insufficiency in the dog. J Small Anim Pract. 1979 Mar;20(3):185–192. doi: 10.1111/j.1748-5827.1979.tb07029.x. [DOI] [PubMed] [Google Scholar]
- Batt R. M., Peters T. J. Analytical subcellular fractionation studies on enterocytes from the jejunum and ileum of the rat and some properties of brush-border alkaline phosphatase. Clin Sci Mol Med. 1978 Aug;55(2):157–165. doi: 10.1042/cs0550157. [DOI] [PubMed] [Google Scholar]
- Batt R. M., Peters T. J. Effects of prednisolone on the small intestinal mucosa of the rat. Clin Sci Mol Med. 1976 Jun;50(6):511–523. doi: 10.1042/cs0500511. [DOI] [PubMed] [Google Scholar]
- Batt R. M., Peters T. J. Subcellular fractionation studies on peroral jejunal biopsies from the dog. Res Vet Sci. 1978 Jul;25(1):94–100. [PubMed] [Google Scholar]
- Batt R. M. Techniques for single and multiple peroral jejunal biopsy in the dog. J Small Anim Pract. 1979 May;20(5):259–268. doi: 10.1111/j.1748-5827.1979.tb06720.x. [DOI] [PubMed] [Google Scholar]
- Batt R. M., Wells G., Peters T. J. The effects of prednisolone on the rat enterocyte at a subcellular level. Clin Sci Mol Med. 1978 Nov;55(5):435–443. doi: 10.1042/cs0550435. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Rousseau G. G., Benson M. C., Garcea R. L., Ito J., Tomkins G. M. Role of DNA and specific cytoplasmic receptors in glucocorticoid action. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1892–1896. doi: 10.1073/pnas.69.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley D. R., Howell K. E., Eichholz A. Solubilization of brush borders of hamster small intestine and fractionation of some of the components. Biochim Biophys Acta. 1975 Jul 3;394(3):361–376. doi: 10.1016/0005-2736(75)90290-4. [DOI] [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. I. Structural and chemical differentiation in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):73–96. doi: 10.1083/jcb.30.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Eichholz A. Studies on the organization of the brush border in intestinal epithelial cells. V. Subfractionation of enzymatic activities of the microvillus membrane. Biochim Biophys Acta. 1968 Aug;163(1):101–107. doi: 10.1016/0005-2736(68)90037-0. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Leiter E. H. Exocrine pancreatic insufficiency syndrome in CBA/J mice. Ultrastructural study. Am J Pathol. 1977 Jan;86(1):17–30. [PMC free article] [PubMed] [Google Scholar]
- Gelehrter T. D. Mechanisms of hormonal induction of enzymes. Metabolism. 1973 Jan;22(1):85–100. doi: 10.1016/0026-0495(73)90033-4. [DOI] [PubMed] [Google Scholar]
- Hill F. W., Osborne A. D., Kidder D. E. Pancreatic degenerative atrophy in dogs. J Comp Pathol. 1971 Jul;81(3):321–330. doi: 10.1016/0021-9975(71)90019-3. [DOI] [PubMed] [Google Scholar]
- Imondi A. R., Stradley R. P., Wolgemuth R. Synthetic peptides in the diagnosis of exocrine pancreatic insufficiency in animals. Gut. 1972 Sep;13(9):726–731. doi: 10.1136/gut.13.9.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong W. K., Seetharam B., Alpers D. H. Effect of exchange exocrine pancreatic insufficiency on small intestine in the mouse. Gastroenterology. 1978 Jun;74(6):1277–1282. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvard D., Maroux S., Vannier C., Desnuelle P. Topological studies on the hydrolases bound to the intestinal brush border membrane. I. Solubilization by papain and Triton X-100. Biochim Biophys Acta. 1975 Jan 28;375(2):235–248. [PubMed] [Google Scholar]
- Louvard D., Semeriva M., Maroux S. The brush-border intestinal aminopeptidase, a transmembrane protein as probed by macromolecular photolabelling. J Mol Biol. 1976 Oct 5;106(4):1023–1035. doi: 10.1016/0022-2836(76)90350-8. [DOI] [PubMed] [Google Scholar]
- MILLER F., PALADE G. E. LYTIC ACTIVITIES IN RENAL PROTEIN ABSORPTION DROPLETS. AN ELECTRON MICROSCOPICAL CYTOCHEMICAL STUDY. J Cell Biol. 1964 Dec;23:519–552. doi: 10.1083/jcb.23.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen W. A., Korsmo H. Enhancement of intestinal sucrase activity in experimental diabetes: the role of intraluminal factors. J Lab Clin Med. 1975 May;85(5):832–837. [PubMed] [Google Scholar]
- Peters T. J., Batt R. M., Heath J. R., Tilleray J. The micro-assay of intestinal disaccharidases. Biochem Med. 1976 Apr;15(2):145–148. doi: 10.1016/0006-2944(76)90041-7. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Seetharam B., Grimme N., Goodwin C., Alpers D. H. Differential sensitivity of intestinal brush border enzymes to pancreatic and lysosomal proteases. Life Sci. 1976 Jan 1;18(1):89–95. doi: 10.1016/0024-3205(76)90278-2. [DOI] [PubMed] [Google Scholar]
- Seymour C. A., Peters T. J. Organelle pathology in primary and secondary haemochromatosis with special reference to lysosomal changes. Br J Haematol. 1978 Oct;40(2):239–253. doi: 10.1111/j.1365-2141.1978.tb03661.x. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Walker W. A., Isselbacher K. J. Uptake and transport of macromolecules by the intestine. Possible role in clinical disorders. Gastroenterology. 1974 Sep;67(3):531–550. [PubMed] [Google Scholar]
- Walker W. A., Wu M., Isselbacher K. J., Bloch K. J. Intestinal uptake of macromolecules. IV.--The effect of pancreatic duct ligation on the breakdown of antigen and antigen-antibody complexes on the intestinal surface. Gastroenterology. 1975 Dec;69(6):1223–1229. [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Plasma membrane synthesis in the macrophage following phagocytosis of polystyrene latex particles. J Biol Chem. 1972 Apr 25;247(8):2439–2446. [PubMed] [Google Scholar]


