Abstract
Active transport of conjugated bile salts, Na-tauro- and Na-glycocholate and D-galactose, was examined in the small intestine of streptozotocin-diabetic rats by an in-vitro technique.
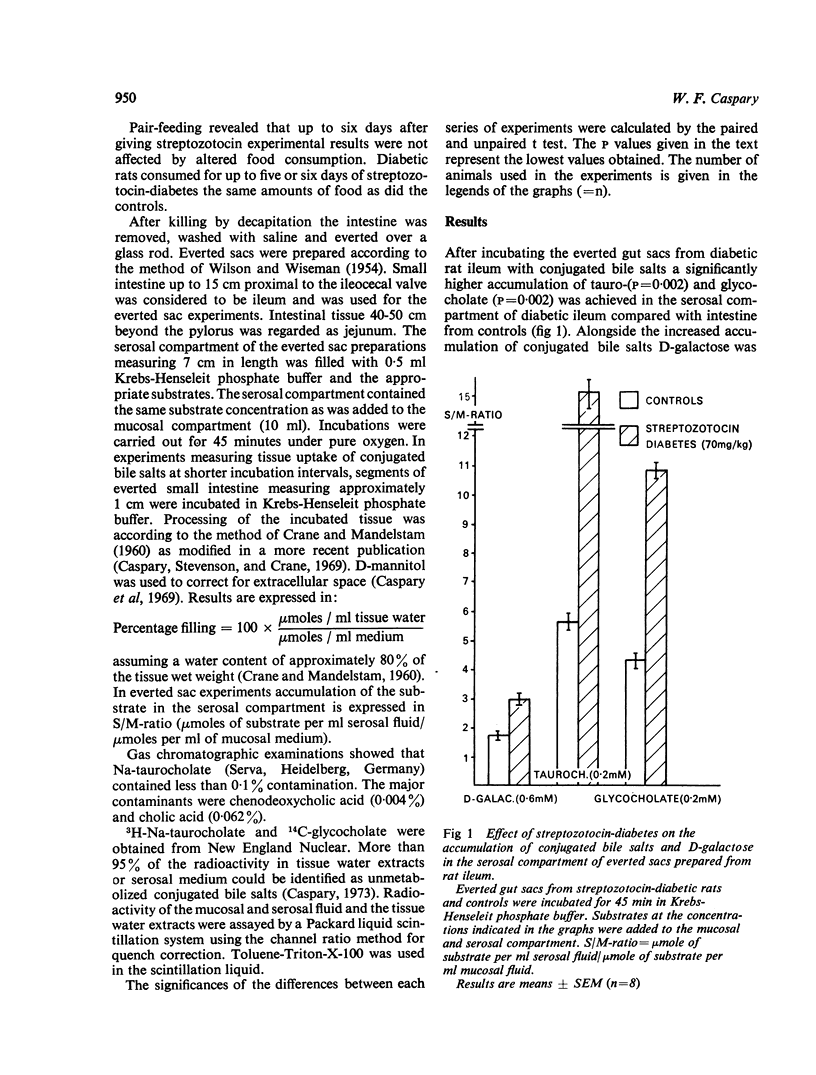
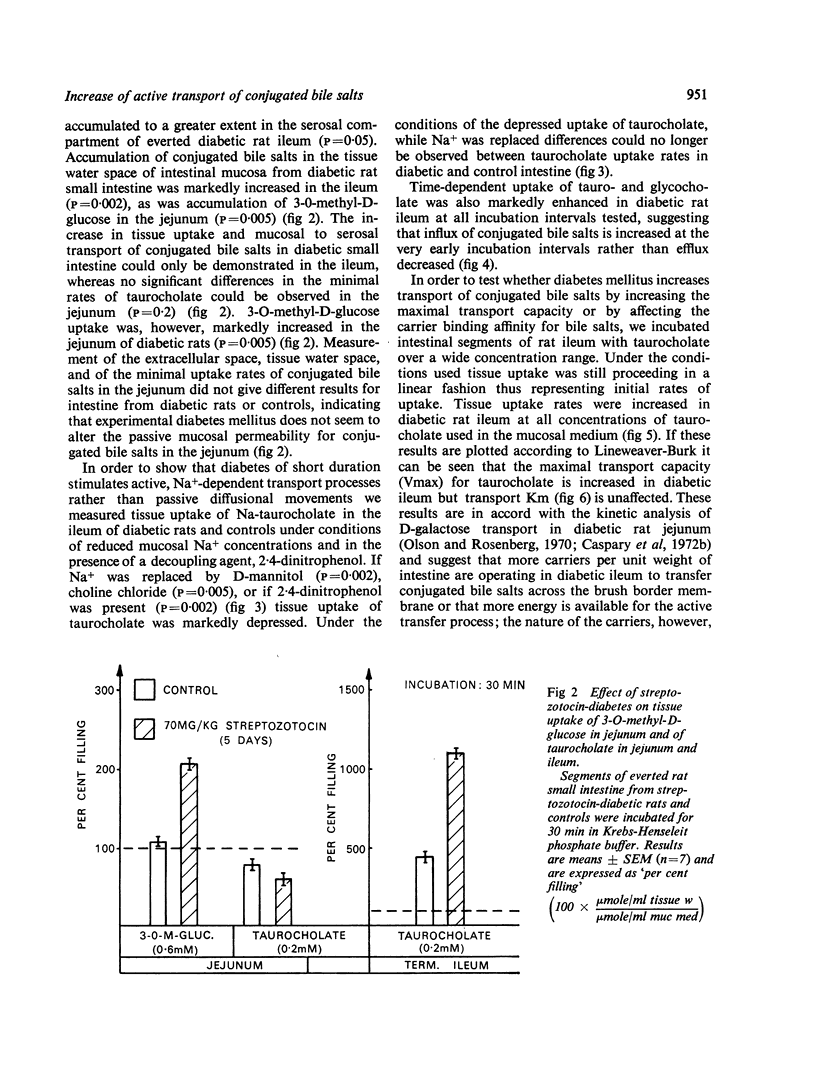
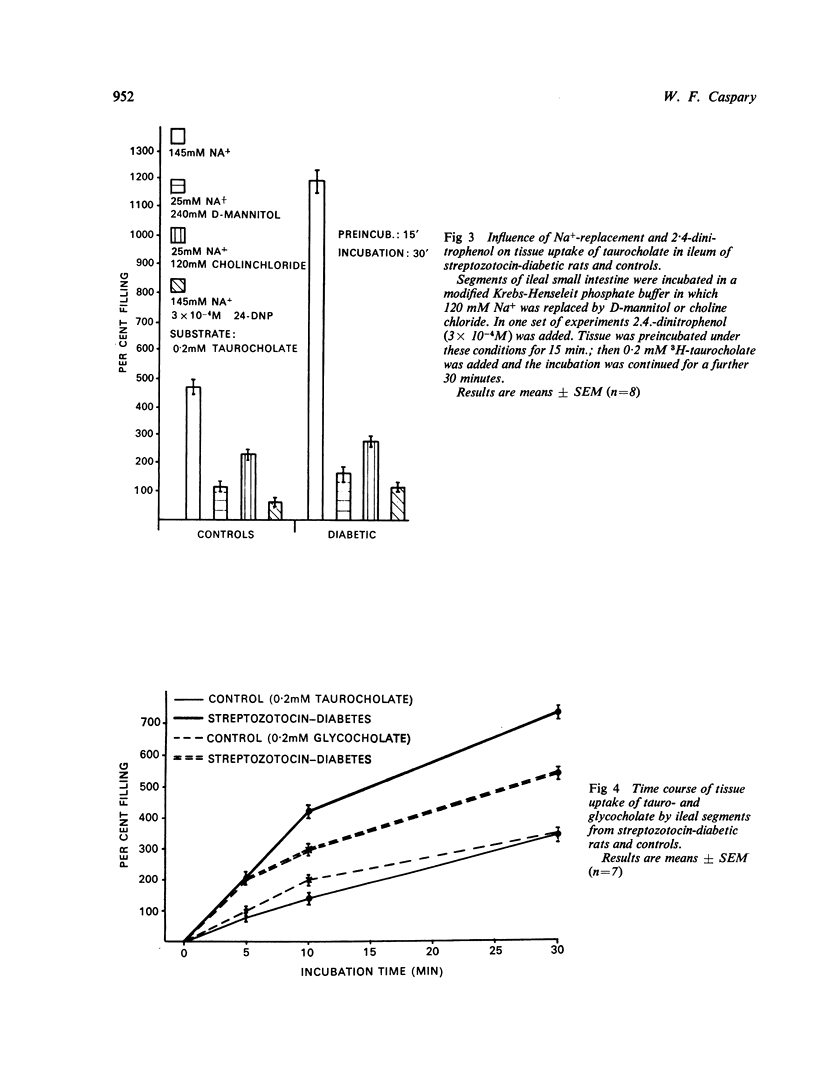
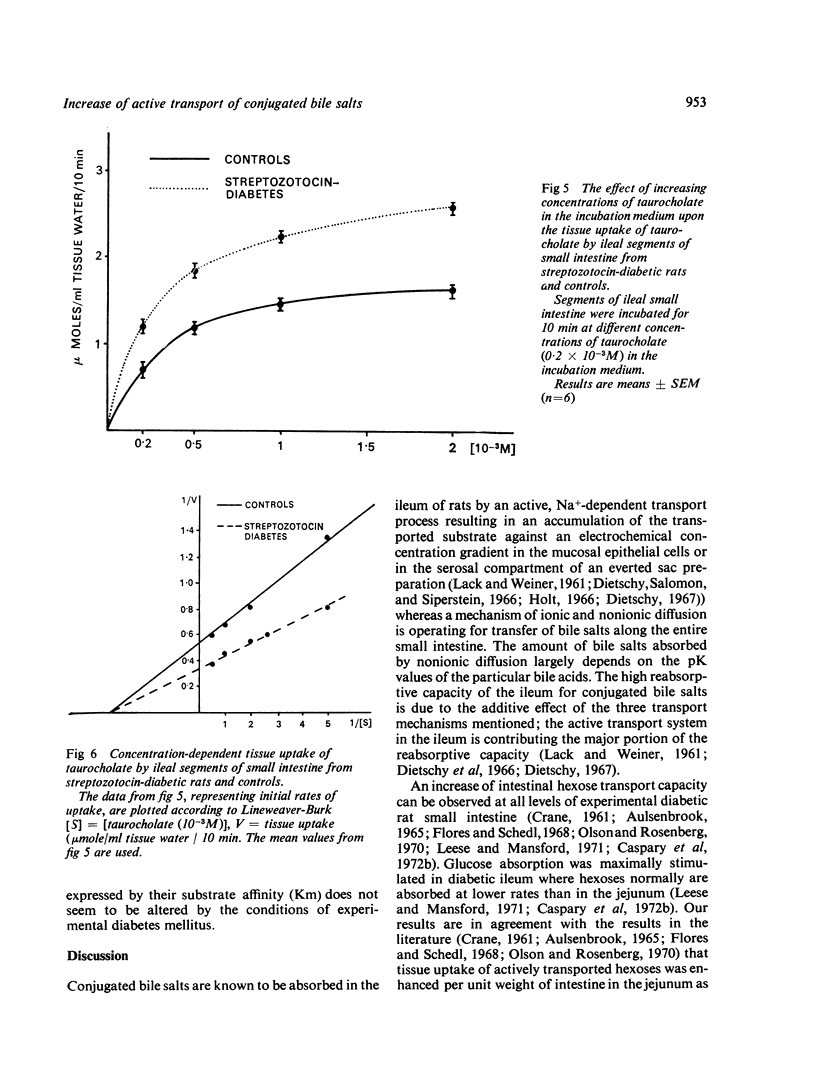
Tissue uptake and mucosal to serosal transport of conjugated bile salts and D-galactose was enhanced in diabetic rat ileum. The minimal transport capacity for conjugated bile salts in the jejunum did not differ between diabetic and control intestine. D-galactose transport and transport of 3-0-methyl-glucose were, however, enhanced in diabetic jejunum as well. Kinetic analysis of the initial uptake rates for conjugated bile salts revealed that the maximal transport capacity (Vmax) was enhanced in diabetic ileum.
In accordance with earlier results on the effect of experimental diabetes mellitus on digestive-absorptive functions it is suggested that experimental diabetes mellitus increases the transport capacity of active, Na+-dependent intestinal transport processes in general.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aulsebrook K. A. Intestinal transport of glucose and sodium: changes in alloxan diabetes and effects of insulin. Experientia. 1965 Jun 15;21(6):346–347. doi: 10.1007/BF02144708. [DOI] [PubMed] [Google Scholar]
- CRANE R. K. An effect of alloxan-diabetes on the active transport of sugars by rat small intestine, in vitro. Biochem Biophys Res Commun. 1961 Apr 28;4:436–440. doi: 10.1016/0006-291x(61)90304-7. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., MANDELSTAM P. The active transport of sugars by various preparations of hamster intestine. Biochim Biophys Acta. 1960 Dec 18;45:460–476. doi: 10.1016/0006-3002(60)91482-7. [DOI] [PubMed] [Google Scholar]
- Caspary W. F., Rhein A. M., Creutzfeldt W. Increase of intestinal brush border hydrolases in mucosa of streptozotocin-diabetic rats. Diabetologia. 1972 Dec;8(6):412–414. doi: 10.1007/BF01212169. [DOI] [PubMed] [Google Scholar]
- Caspary W. F., Stevenson N. R., Crane R. K. Evidence for an intermediate step in carrier-mediated sugar translocation across the brush border membrane of hamster small intestine. Biochim Biophys Acta. 1969 Oct 14;193(1):168–178. doi: 10.1016/0005-2736(69)90070-4. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M. Effects of bile salts on intermediate metabolism of the intestinal mucosa. Fed Proc. 1967 Nov-Dec;26(6):1589–1598. [PubMed] [Google Scholar]
- Dietschy J. M., Salomon H. S., Siperstein M. D. Bile acid metabolism. I. Studies on the mechanisms of intestinal transport. J Clin Invest. 1966 Jun;45(6):832–846. doi: 10.1172/JCI105399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores P., Schedl H. P. Intestinal transport of 3-O-methyl-D-glucose in the normal and alloxan-diabetic rat. Am J Physiol. 1968 Apr;214(4):725–729. doi: 10.1152/ajplegacy.1968.214.4.725. [DOI] [PubMed] [Google Scholar]
- HOLT P. R. INTESTINAL ABSORPTION OF BILE SALTS IN THE RAT. Am J Physiol. 1964 Jul;207:1–7. doi: 10.1152/ajplegacy.1964.207.1.1. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F. A physicochemical approach to the intraluminal phase of fat absorption. Gastroenterology. 1966 Jan;50(1):56–64. [PubMed] [Google Scholar]
- Jervis E. L., Levin R. J. Anatomic adaptation of the alimentary tract of the rat to the hyperphagia of chronic alloxan-diabetes. Nature. 1966 Apr 23;210(5034):391–393. doi: 10.1038/210391a0. [DOI] [PubMed] [Google Scholar]
- KERSHAW T. G., NEAME K. D., WISEMAN G. The effect of semistarvation on absorption by the rat small intestine in vitro and in vivo. J Physiol. 1960 Jun;152:182–190. doi: 10.1113/jphysiol.1960.sp006480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACK L., WEINER I. M. In vitro absorption of bile salts by small intestine of rats and guinea pigs. Am J Physiol. 1961 Feb;200:313–317. doi: 10.1152/ajplegacy.1961.200.2.313. [DOI] [PubMed] [Google Scholar]
- Leese H. J., Mansford K. R. The effect of insulin and insulin deficiency on the transport and metabolism of glucose by rat small intestine. J Physiol. 1971 Feb;212(3):819–838. doi: 10.1113/jphysiol.1971.sp009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low-Beer T. S., Pomare E. W. Regulation of bile salt pool size in man. Br Med J. 1973 May 12;2(5862):338–340. doi: 10.1136/bmj.2.5862.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill L. K., Hamilton J. R. The effect of fasting on disaccharidase activity in the rat small intestine. Pediatrics. 1971 Jan;47(1):65–72. [PubMed] [Google Scholar]
- Northfield T. C., Hofmann A. F. Biliary lipid secretion in gallstone patients. Lancet. 1973 Apr 7;1(7806):747–748. doi: 10.1016/s0140-6736(73)92130-2. [DOI] [PubMed] [Google Scholar]
- Olsen W. A., Rogers L. Jejunal sucrase activity in diabetic rats. J Lab Clin Med. 1971 May;77(5):838–842. [PubMed] [Google Scholar]
- Olsen W. A., Rosenberg I. H. Intestinal transport of sugars and amino acids in diabetic rats. J Clin Invest. 1970 Jan;49(1):96–105. doi: 10.1172/JCI106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl H. P., Wilson H. D. Effects of diabetes on intestinal growth in the rat. J Exp Zool. 1971 Apr;176(4):487–495. doi: 10.1002/jez.1401760410. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol. 1954 Jan;123(1):116–125. doi: 10.1113/jphysiol.1954.sp005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M. J., Baker R. D. Effect of taurocholate on electrical potential difference across rat small intestine. Life Sci I. 1972 Apr 15;11(8):375–386. doi: 10.1016/0024-3205(72)90047-1. [DOI] [PubMed] [Google Scholar]
- Younoszai M. K., Schedl H. P. Effect of diabetes on intestinal disaccharidase activities. J Lab Clin Med. 1972 Apr;79(4):579–586. [PubMed] [Google Scholar]


