Abstract
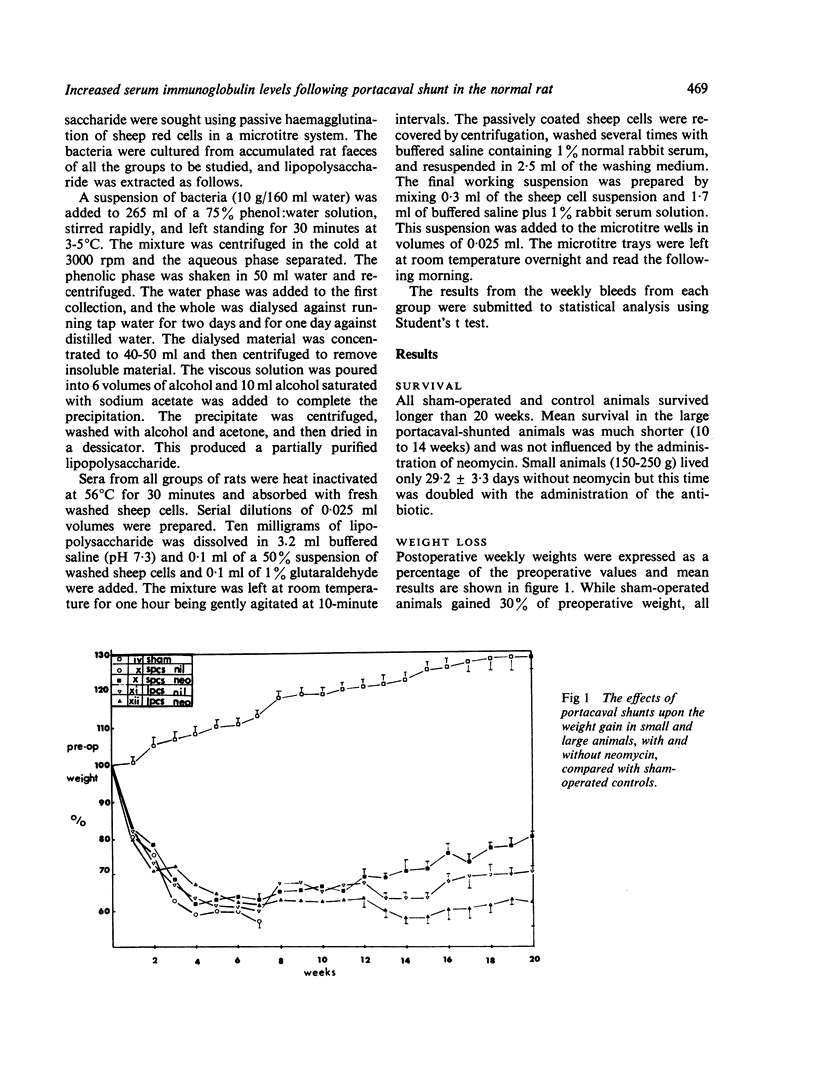
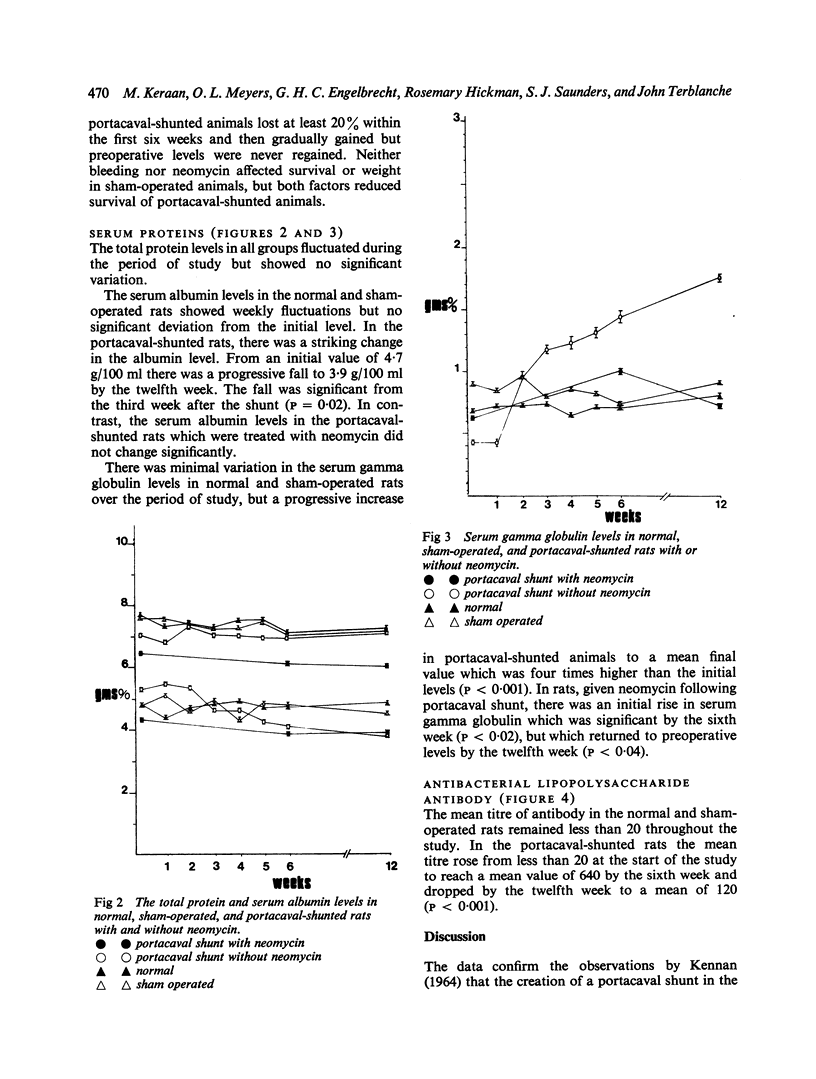
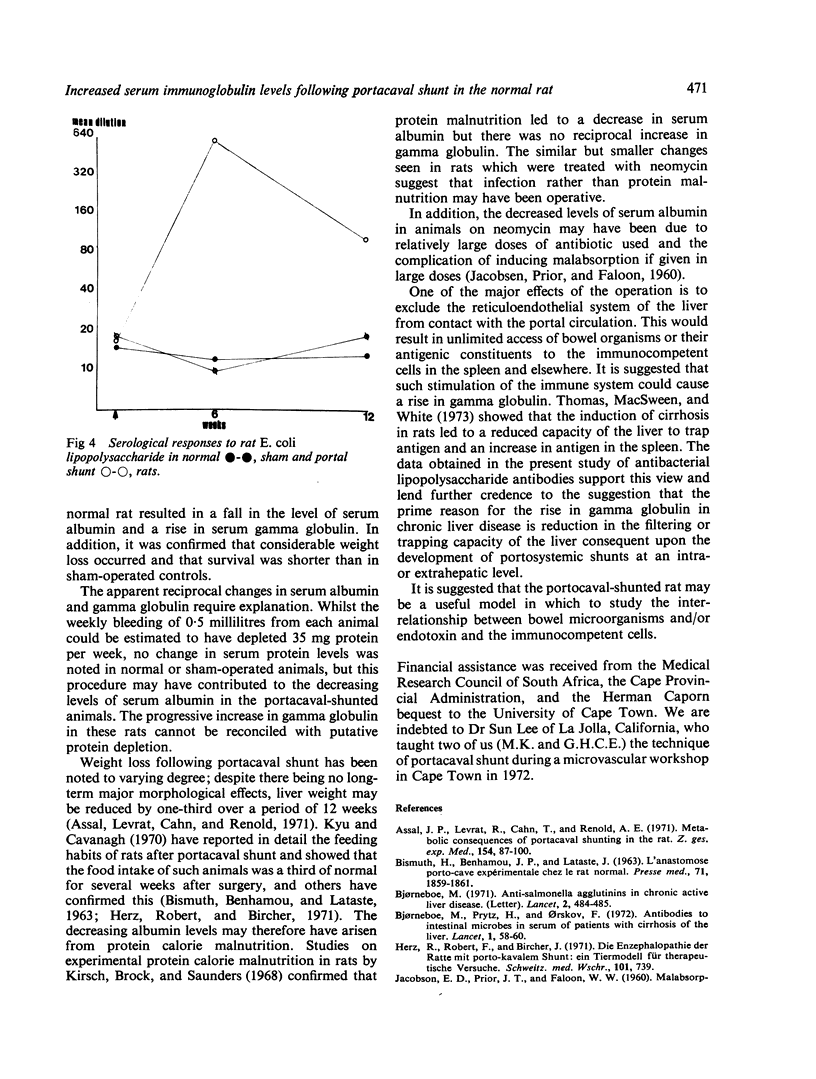
Normal rats subjected to end-to-side portacaval shunt showed decreased survival and weight gain, a progressive fall in serum albumin and reciprocal rise in serum gamma globulin when compared with sham-operated controls for 12 weeks. Antibacterial lipopolysaccharide antibody was detected in significant titre at the sixth and twelfth weeks.
It is suggested that the elevated levels of gamma globulin and reversal of albumin/globulin ratios noted in these animals may represent an immune response to bacterial lipopolysaccharides released into the systemic circulation as a result of the portacaval shunt. The hyperglobulinaemia of cirrhosis in human subjects may have a similar aetiology.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assal J. P., Levrat R., Cahn T., Renold A. E. Metabolic consequences of portacaval shunting in the rat. Effects on weight, food intake, intestinal absorption and hepatic morphology. Z Gesamte Exp Med. 1971;154(2):87–100. doi: 10.1007/BF02048382. [DOI] [PubMed] [Google Scholar]
- BISMUTH H., BENHAMOU J. P., LATASTE J. L'ANASTOMOSE PORTO-CAVE EXP'ERIMENTALE CHEZ LE RAT NORMAL. TECHNIQUE ET R'ESULTATS PR'ELIMINAIRES. Presse Med. 1963 Sep 25;71:1859–1861. [PubMed] [Google Scholar]
- Bjorneboe M. Anti-salmonella agglutinins in chronic active liver disease. Lancet. 1971 Aug 28;2(7722):484–485. doi: 10.1016/s0140-6736(71)92644-4. [DOI] [PubMed] [Google Scholar]
- Bjorneboe M., Prytz H., Orskov F. Antibodies to intestinal microbes in serum of patients with cirrhosis of the liver. Lancet. 1972 Jan 8;1(7741):58–60. doi: 10.1016/s0140-6736(72)90060-8. [DOI] [PubMed] [Google Scholar]
- Herz R., Robert F., Bircher J. Die Enzephalopathie der Ratte mit porto-kavalem Shunt: ein Tiermodell für therapeutische Versuche. Schweiz Med Wochenschr. 1971 May 22;101(20):739–739. [PubMed] [Google Scholar]
- JACOBSON E. D., PRIOR J. T., FALOON W. W. Malabsorptive syndrome induced by neomyclin: morphologic alterations in the jejunal mucosa. J Lab Clin Med. 1960 Aug;56:245–250. [PubMed] [Google Scholar]
- KENNAN A. L. CHANGES IN PLASMA PROTEINS AFTER PORTACAVAL SHUNT IN THE RAT. Proc Soc Exp Biol Med. 1964 Jun;116:543–544. doi: 10.3181/00379727-116-29301. [DOI] [PubMed] [Google Scholar]
- Kirsch R. E., Brock J. F., Saunders S. J. Experimental protein-calorie malnutrition. Am J Clin Nutr. 1968 Aug;21(8):820–826. doi: 10.1093/ajcn/21.8.820. [DOI] [PubMed] [Google Scholar]
- Kyu M. H., Cavanagh J. B. Some effects of porto-caval anastomosis in the male rat. Br J Exp Pathol. 1970 Apr;51(2):217–227. [PMC free article] [PubMed] [Google Scholar]
- Paronetto F. Immunologic aspects of liver diseases. Prog Liver Dis. 1970;3:299–318. [PubMed] [Google Scholar]
- Thomas H. C., McSween R. N., White R. G. Role of the liver in controlling the immunogenicity of commensal bacteria in the gut. Lancet. 1973 Jun 9;1(7815):1288–1291. doi: 10.1016/s0140-6736(73)91300-7. [DOI] [PubMed] [Google Scholar]
- Triger D. R., Alp M. H., Wright R. Bacterial and dietary antibodies in liver disease. Lancet. 1972 Jan 8;1(7741):60–63. doi: 10.1016/s0140-6736(72)90061-x. [DOI] [PubMed] [Google Scholar]
- WIEME R. J. An improved technique of agar-gel electrophoresis on microscope slides. Clin Chim Acta. 1959 May;4(3):317–321. doi: 10.1016/0009-8981(59)90096-8. [DOI] [PubMed] [Google Scholar]


