Abstract
Presenilins (PS1/PS2) regulate proteolysis of β-amyloid precursor protein (βAPP) and affect its intracellular trafficking. Here, we demonstrate that a PS1-interacting protein, phospholipase D1 (PLD1), affects intracellular trafficking of βAPP. Overexpression of PLD1 in PS1wt cells promotes generation of βAPP-containing vesicles from the trans-Golgi network. Conversely, inhibition of PLD1 activity by 1-butanol decreases βAPP trafficking in both wt and PS1-deficient cells. The subcellular localization of PLD1 is altered, and PLD enzymatic activity is reduced in cells expressing familial Alzheimer’s disease (FAD) PS1 mutations compared with PS1wt cells. Overexpression of wt, but not catalytically inactive, PLD1 increases budding of βAPP-containing vesicles from the trans-Golgi network in FAD mutant cells. Surface delivery of βAPP is also increased by PLD1 in these cells. The impaired neurite outgrowth capacity in FAD mutant neurons was corrected by introducing PLD1 into these cells. The results indicate that PLD1 may represent a therapeutic target for rescuing compromised neuronal function in AD.
Keywords: β-amyloid precursor protein, intracellular trafficking, axonal growth, neurite branching, trans-Golgi network
The distribution of β-amyloid precursor protein (βAPP) between the trans-Golgi network (TGN) and the cell surface may determine the relative generation of sβAPPα versus β-amyloid (1–5). Using a cell-free vesicle-trafficking reconstitution system derived from neuroblastoma cells, we showed previously that presenilin-1 (PS1), a major component of γ-secretase, selectively affects βAPP budding from the TGN and the endoplasmic reticulum (ER) (6). Familial Alzheimer’s disease (FAD) PS1 mutations impair the budding of vesicles containing βAPP, which may partially account for increased βAPP processing via β- and γ-secretases.
Protein trafficking within the secretory pathway is regulated by a rigorously orchestrated sequence of events. Recruitment of cytosolic factors and coat proteins to membranes, and changes in lipid composition that promote membrane curvature and protein anchoring, are necessary for vesiculation (7, 8). Estradiol-promoted βAPP trafficking depends on the recruitment of cytosolic trafficking factor Rab11 to the TGN (3).
Phospholipase D (PLD), a phospholipid-modifying enzyme, catalyzes the hydrolysis of phosphatidylcholine to generate phosphatidic acid (PA) (8–10). PLD has been shown to regulate membrane trafficking events, such as the release of secretory vesicles from the TGN (11), endocytosis (12) and exocytosis (13), and actin dynamics (14). In this study, the effects of PLD1 on βAPP trafficking have been investigated. Of particular interest, up-regulation of PLD1 in FAD-linked PS1 mutant cells rescues impaired βAPP trafficking from the TGN to the plasma membrane and corrects FAD-related defects in neurite outgrowth capacity. In a companion paper (15), we report that PLD1 interacts with PS1 and, through a mechanism independent of its effect on βAPP trafficking, disrupts the γ-secretase complex and inhibits γ-secretase activity.
Results
PLD1 Regulates βAPP Trafficking.
We investigated whether PLD1 might be involved in regulation of βAPP intracellular trafficking. The effect of PLD1 on βAPP trafficking was assessed in both PS1wt and PS1-deficient cells. Consistent with our previous observations (6), the rate of formation of βAPP-containing vesicles from the TGN in PS1−/− fibroblasts was increased compared with PS1wt cells (Fig. 1a). Overexpression of PLD1 in PS1wt cells increased βAPP trafficking from the TGN to a rate comparable with that seen in PS1−/−-deficient cells. However, PLD1 overexpression failed to increase the number of βAPP-containing vesicles budded from the TGN in PS1−/− fibroblasts, probably because of a maximal rate of βAPP-containing vesicle trafficking in PS1-null cells. Inhibition of PLD1 catalytic activity by 1-butanol (an inhibitor of PLD-catalyzed formation of PA) (11, 16), resulted in decreased βAPP trafficking from the TGN in both PS1wt and PS1−/− cells (Fig. 1b). As a control, tertiary butanol, which does not affect PLD activity (12), caused little change in βAPP budding from the TGN. These results demonstrate that PLD1 can affect βAPP trafficking through a PS1-independent mechanism.
Fig. 1.
PLD1 regulates βAPP trafficking. (a) βAPP-containing vesicle budding from the TGN was measured in PS1wt and PS1−/− fibroblasts transfected with PLD1 or mock cDNA. (b) βAPP-containing vesicle budding from the TGN was measured in PS1wt and PS1−/− fibroblasts treated with 3-butanol or 1-butanol. ∗, P < 0.01; ∗∗, P < 0.001, Student’s t test.
FAD-Linked PS1 Mutation Changes the Subcellular Localization of PLD1 and Reduces the Catalytic Activity of PLD.
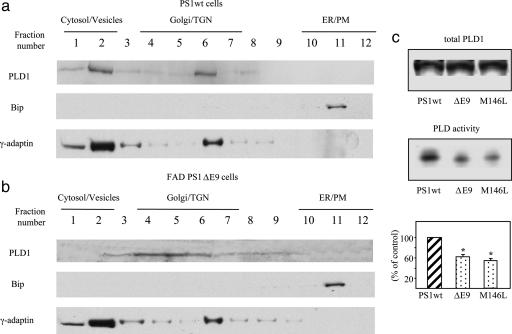
In wt cells, PLD1 was localized in cytosol/light vesicles (likely endosomes) and Golgi/TGN fractions, and was codistributed with a marker for Golgi/TGN and light vesicles, γ-adaptin, but not with Bip, an ER marker (Fig. 2a). The subcellular localization of PLD1 changed in cells expressing a FAD-linked PS1 mutant, ΔE9, being limited to the Golgi/TGN with little found in cytosol/light vesicles (Fig. 2b). Furthermore, PLD catalytic activity in cells expressing a FAD-linked PS1 mutant (ΔE9 or M146L) was significantly lower than that in wt counterparts (Fig. 2c). These results suggest that both PLD1 trafficking/recycling and PLD-mediated PA synthesis are impaired by FAD-linked PS1 mutations.
Fig. 2.
FAD-linked PS1 mutation changes the subcellular localization of PLD1 and reduces the catalytic activity of PLD. N2a cells expressing PS1wt (a) or FAD mutant ΔE9 (b) were homogenized, and postnuclear supernatants were fractioned on an equilibrium floatation sucrose gradient. Thirty-microliter samples from each fraction were subjected to 8% SDS/PAGE, followed by immunoblotting with anti-PLD1 antibody AE596. Organelle markers were analyzed by immunoblotting. Bip, ER marker; γ-adaptin, vesicle/Golgi marker. (c) PLD total protein levels and activities were measured in N2a cells expressing PS1wt, ΔE9, or M146L variants. Total PLD1 protein levels were determined by immunoblotting with PLD1 antibody. Relative levels of PLD activity were determined by quantifying the intensity of phospho-butanol bands. (Bottom) PLD activity is expressed as percent. ∗, P < 0.01, Student’s t test.
PLD1 Rescues the Impaired Budding of βAPP-Containing Vesicles in FAD-Linked PS1 Mutant Cells.
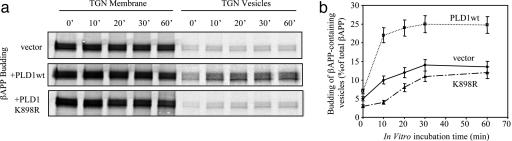
We previously showed that FAD PS1 mutations impair the budding of vesicles containing βAPP (6). Overexpression of wt PLD1 in PS1ΔE9 N2a cells increased βAPP vesicle budding from the TGN (Fig. 3). In contrast, overexpression of catalytically inactive PLD1-K898R (17), which is impaired in catalyzing hydrolysis of phosphatidylcholine to form PA, failed to stimulate βAPP trafficking in ΔE9 cells (Fig. 3). These results indicate that catalytically active PLD1 plays a crucial role in PLD1-regulated βAPP vesicle biogenesis.
Fig. 3.
PLD1 rescues the impaired budding of βAPP-containing vesicles in FAD-linked PS1 mutant cells. (a) Kinetics of βAPP-containing vesicle formation was analyzed in N2a PS1ΔE9 cells transiently transfected with PLD1 wt cDNA or PLD1 K898R cDNA and compared with mock transfections. (b) The amount of βAPP in budded vesicles was expressed as the percentage of total βAPP.
Interestingly, another cytosolic factor, Rab11, shown to be important in stimulation of TGN vesicle biogenesis by estrogen (3), had no effect on βAPP trafficking in PS1wt or FAD mutant cells (Fig. 6, which is published as supporting information on the PNAS web site), indicating specificity of the PLD1-mediated effects.
PLD1 Accelerates the Slowed Surface Delivery of βAPP in FAD Mutant Cells.
To determine whether the PLD1-induced increase in budding of βAPP-containing vesicles from the TGN of FAD mutant cells would lead to changes in βAPP surface delivery, surface-bound βAPP was detected by immunofluorescent staining. Overexpression of PLD1 significantly increased the amount of surface-bound βAPP compared with controls both in N2a cells expressing PS1ΔE9 (Fig. 4a) and in primary neurons from PS1 M146V knockin mice (data not shown). As a control, the level of total surface glycoproteins was determined by staining for Vicia villosa agglutinin (VVA) (6, 18) and was shown to be unaffected by overexpression of PLD1. Surface biotinylation confirmed that, in ΔE9 mutant cells, overexpression of PLD1 increased the amount of surface βAPP (Fig. 4b).
Fig. 4.
PLD1 accelerates the slowed surface delivery of βAPP in FAD mutant cells. (a) N2a PS1ΔE9 cells overexpressing PLD1 or mock transfected cells were incubated with antibody 6E10 (1:100) at 4°C to label cell surface βAPP (red). FITC-conjugated VVA was used to stain all surface glycoproteins (green). Immunofluorescence was visualized by confocal microscopy. (b) Cell surface proteins were biotinylated as described in Methods. One percent of total protein lysate was loaded as input (lanes labeled as total βAPP). Graph shows means ± SE of three experiments. ∗∗, P < 0.001, Student’s t test. (Scale bar, 10 μm.)
PLD1 Corrects the Impaired Neurite Outgrowth/Branching Capacity in FAD Mutant Neurons.
We next examined the effect of PLD1 on axonal transport of βAPP in primary neurons. Neurons expressing a PS1 FAD mutation exhibit a profound decrease of surface βAPP at axonal terminals (6). Full-length βAPP has been implicated in a number of physiological functions, such as synapse formation, growth cone outgrowth, and axon guidance (19). Therefore, we reasoned that the impaired delivery of βAPP to the cell surface at axonal terminals in FAD PS1 mutant neurons might contribute to the compromised synaptic function and deregulated neurite growth and stability observed in FAD-linked PS1 mutations. Indeed, it was found that, when FAD-linked PS1M146V cortical neurons were cultured on a substrate of purified CNS myelin, known to be inhibitory for neurite outgrowth (20), the neurite growth capacity in mutant neurons was dramatically reduced compared to wt counterparts (Fig. 5). There was a 59% reduction in neurite length and a 48% reduction in branching (the number of total processes projecting from neurons more than two cell bodies in length). When PLD1wt was introduced into the PS1 mutant neurons, neurite length was largely restored, and branching was partially restored (Fig. 5). However, there was no effect on neurite length or branching when catalytically inactive PLD1 K898R was used. Together, these data suggest that PLD1 is able to rescue the impaired neurite outgrowth/branching capacity of FAD-linked PS1 mutant neurons and that this PLD1 effect is likely due to its catalytic activity (production of PA) and promotion of βAPP axonal transport.
Fig. 5.
PLD1 corrects the impaired neurite outgrowth/branching capacity in FAD mutant neurons. (a) Embryonic day 17 cortical neurons (PS1wt or FAD-linked PS1 mutant M146V) were transfected with PLD1wt, PLD1 K898R, or control GFP and then plated onto myelin slides for neurite outgrowth assays, followed by immunolabeling for GAP43. (b) Quantification of the longest neurites from 200 to 300 positively double-immunolabeled neurons (GAP43 and HA-PLD1 or GAP43 and GFP control) and quantification of the number of total processes (more than two cell bodies in length) projecting from each positive neuron. ∗, P < 0.01; ∗∗, P < 0.001, Student’s t test. (Scale bar, 10 μm.)
Discussion
We report here that FAD-linked PS1 mutants sequester PLD1 on the Golgi/TGN, depleting it from cytosolic vesicles and reducing its catalytic activity and its generation of PA and choline. Overexpression of wt PLD1 in FAD PS1 mutant cells rescues the impaired βAPP trafficking from the TGN, accelerates the slowed surface delivery of βAPP, and corrects impaired neurite outgrowth/branching. The effects of PLD1 were seen with wt PLD1 but not with a catalytically inactive form of PLD1 (K898R). Consistent with our observations, others have shown a signaling role for catalytically active PLD in regulating membrane traffic and actin dynamics (14). An increased concentration of PA, the product of PLD catalytic activity, in the donor membrane has been shown to be crucial for vesicle biogenesis, perhaps because of increased lipid fluidity and the tendency of PA to attract cytosolic factors, such as coat proteins, through its negative charge (21).
Many regulatory functions have been described for PLD1. For example, it was reported that the localization of PLD1 at presynaptic membrane zones, such as axonal neurites and growth cone-like structures, plays a crucial role in controlling the number of functional release sites and modulating neurotransmitter release (13). PLD activation also plays an important role in controlling the actin cytoskeleton and cell migration, which are crucial for regulating synaptic function (22). It is important to note that, in early Alzheimer’s disease (AD), synaptic pathology is more prominent than neuronal loss (23). In FAD-linked PS1 variants, impaired delivery of full-length βAPP to the cell surface at axonal terminals may contribute to disturbances in neurite initiation, elongation and branching, and synaptic plasticity. Collectively, our findings indicate that PLD1 increases surface delivery of βAPP at axonal terminals and rescues impaired axonal growth and neurite branching in FAD PS1 mutant neurons. It is also possible that growth-promoting effects of PLD1 may be partially mediated through its modulation of Rho pathways by activation of PLD2, which has also been implicated in neurite outgrowth (24, 25), or through activation of phosphatidylinositol-4-phosphate 5-kinase (PIP5K) to generate phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], a cofactor for both PLD1 and PLD2 (26).
It has been reported that, in AD brains, the phosphoinositol hydrolysis pathways are dysregulated, resulting in reduced PKC levels and activity, and a reduced number of receptor sites for inositol 1,4,5-trisphosphate (IP3) (27). In addition, disordered metabolism of membrane phospholipids has been reported in AD (28, 29). Our data further suggest that defects in PLD-related metabolism may contribute to AD pathogenesis. Thus, a FAD-linked PS1 mutant sequestered PLD1 on the Golgi/TGN membranes and depleted it from the cytosolic vesicles. This phenotype may disrupt the intracellular pathway catalyzed by PLD1 to generate choline for acetylcholine synthesis and contribute to the selective vulnerability of cholinergic neurons in AD.
The present study has demonstrated that PLD1 regulates intracellular trafficking of βAPP. In a companion report (15), it is demonstrated that PLD1, through a mechanism independent of βAPP trafficking, compromises the integrity of the γ-secretase complex and thereby inhibits β-amyloid formation. These combined actions of PLD1 suggest the development of therapeutic approaches for restoring neuronal dysfunctions in AD.
Methods
Cell Lines.
Mouse N2a neuroblastoma cells doubly transfected with cDNAs encoding human βAPP harboring the “Swedish” mutant (βAPPswe) and human PS1wt or a FAD PS1 mutant ΔE9 (30, 31) were maintained in medium containing 50% DMEM, 50% OptiMEM, supplemented with 5% FBS, antibiotics, and 200 μg/ml G418 (Invitrogen). Immortalized PS1−/− fibroblasts (32) were maintained in DMEM supplemented with 10% FBS and antibiotics.
Neuronal Cultures.
Embryonic cortical neurons (embryonic day 17) from PS1 knockout mouse embryos, rescued with comparable levels of expression of either mouse PS1wt or homozygous FAD-linked PS1 M146V knockin (33), were dissociated and cultured on poly-l-lysine (Sigma) precoated tissue culture dishes for 72 h. They were then transfected with hemagglutinin (HA)-PLD1 cDNA (wt or K898R) or GFP cDNA and transferred (≈5 × 104 cells per chamber) onto eight-chamber slides (Lab-Tek) coated with a substrate of purified CNS myelin. A neurite outgrowth assay was performed as described, and neurons were immunolabeled for growth-associated protein 43 (GAP43) (34). The length of the longest neurite extending from each neuron, as well as the number of total processes (more than two cell bodies) projecting from individual neurons, was quantified by an Oncor imaging analysis system for the first 200–300 neurons encountered when scanning the slide in a systematic manner (34). Only positively double-immunolabeled cells (GAP43 and HA or GAP43 and GFP) were examined.
Sucrose Gradient Fractionation.
To separate and enrich TGN and ER membranes, cells were homogenized by using a stainless-steel ball-bearing cell cracker, and cell lysates were fractionated by using sucrose density gradient centrifugation as described (1, 35). PLD1 in each fraction was determined by immunoblotting with antibody AE596 (36).
PLD Activity Assay.
N2a cells expressing PS1wt or FAD-linked PS1 mutations were labeled with [3H]myristate and then washed with isotonic Tris-saline buffer and rapidly treated with 500 μl of methanol:6N HCl (50:2). Lipids were then extracted by addition of 0.5 ml of CHCl3. Phase separation was obtained by adding 155 μl of 1 M NaCl. The organic phase was reextracted with 120 μl of 1 M NaCl, 350 μl of H2O, and 155 μl of methanol and recovered. Samples were then normalized for total radioactivity counts (cpm), and phospholipid metabolites were characterized by TLC. Relative levels of PLD activity were determined by measuring the intensity of corresponding phospho-butanol bands.
Preparation of Permeabilized N2a Cells.
To assay βAPP trafficking from the TGN, fibroblasts (PS1wt or PS1−/−) transfected with PLD1 cDNA or mock cDNA were pulse-labeled with [35S]methionine (500 μCi/ml) (1 Ci = 37 GBq) for 15 min at 37°C, washed with PBS (prewarmed to 20°C), and chased for 2 h at 20°C in prewarmed complete media. Cells were then permeabilized as described (6). Permeabilized cells (cell-free system) were washed and incubated in a final volume of 300 μl containing 2.5 mM MgCl2, 0.5 mM CaCl2, 110 mM KCl, cytosol (30 μg protein) prepared from N2a cells (3, 37) with an energy-regenerating system consisting of 1 mM ATP, 0.02 mM GTP, 10 mM creatine phosphate, 80 μg/ml creatine phosphokinase, and a complete protease inhibitor mixture. Alternatively, 0.3% 3-butanol or 1-butanol was included in the cell-free system derived from PS1wt or PS1−/− fibroblasts. Similarly, N2a PS1ΔE9 cells transfected with PLD1 wt or K898R cDNA fibroblast cells (PS1wt or PS1−/−) were permeabilized for cell-free budding assay preparations. Incubations were then carried out at 37°C for various periods (15–90 min) to observe the kinetics of βAPP trafficking.
Measurement of Nascent Secretory Vesicles in Permeabilized Cells and Immunoprecipitation.
After incubation of cell-free systems, vesicle and membrane fractions were separated by centrifugation at 1.14 × 104 × g for 30 s at 4°C in a Brinkmann centrifuge. Vesicle (supernatant) and membrane (pellet) fractions were diluted with immunoprecipitation buffer (50 mM Tris·HCl, pH 8.8/150 mM NaCl/6 mM EDTA/2.5% Triton X-100/5 mM methionine/5 mM cysteine/1 mg/ml BSA), immunoprecipitated using anti-βAPP C-terminal antibody 369 (4, 38), and analyzed by SDS/PAGE. Each experiment was performed at least three times. Band intensities were analyzed and quantified by using NIH imagequant software, version 1.61.
Immunofluorescence Confocal Microscopy.
For staining of surface βAPP, N2a cells grown in chamber slides were incubated at 4°C with primary antibody 6E10 (diluted 1:100 in growth medium; Signet Laboratories, Dedham, MA) for 1 h without fixation or permeabilization. After incubation with secondary antibodies and FITC-conjugated VVA (1:100; Vector Laboratories), cells were fixed with 4% formaldehyde at room temperature for 15 min. For neurite length and branching analysis, cultured neurons were fixed with 4% formaldehyde for 15 min twice, followed by permeabilization with ice-cold methanol. Cells were then incubated with primary antibody against GAP43 (1:4,000) and anti-HA antibody (50 ng/ml; Roche Diagnostics) at 4°C overnight. Immunofluorescence staining was examined by confocal microscopy (LSM510; Zeiss).
Biotinylation and Detection of Cell Surface βAPP at Steady State.
N2a PS1ΔE9 cells transiently transfected with PLD1 wt or mock cDNA were incubated for 30 min at 4°C with 0.5 mg·ml−1 sulfo-N-hydroxysuccinimide biotin (Pierce) to biotinylate cell surface proteins. Cells were lysed and biotinylated, and nonbiotinylated proteins were separated into two fractions by binding to streptavidin agarose beads (Pierce). βAPP from each fraction was analyzed by SDS/PAGE and immunoblotting with 6E10 antibody.
Supplementary Material
Acknowledgments
We thank Michael Frohman of the State University of New York, Stony Brook for generously providing plasmids encoding HA-tagged PLD1 wt and K898R and critically reading the manuscript. We also thank Marie T. Filbin of Hunter College of the City University of New York for assistance on neurite outgrowth imaging analysis. This work was supported by National Institutes of Health (NIH) Grants DK21860 (to D.S.), NCI CA46677 (to D.A.F.), NS046673 (to H.X.), and AG09464 (to P.G.); a Veterans Affairs Merit Award (to F.S.G.); an Alzheimer’s Association Investigator-Initiated Research Grant award (to H.X. and D.C.); and the Fisher Center for Alzheimer’s Research Foundation and the F. M. Kirby Foundation, Inc. D.C. is a recipient of NIH National Research Service Award Fellowship 5F32AG023431.
Abbreviations
- βAPP
β-amyloid precursor protein
- TGN
trans-Golgi network
- PS1
presenilin-1
- ER
endoplasmic reticulum
- PLD
phospholipase D
- PA
phosphatidic acid
- AD
Alzheimer’s disease
- FAD
familial AD
- VVA
Vicia villosa agglutinin
- GAP43
growth-associated protein 43
- HA
hemagglutinin
- wt
wild type.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Greenfield J. P., Tsai J., Gouras G. K., Hai B., Thinakaran G., Checler F., Sisodia S. S., Greengard P., Xu H. Proc. Natl. Acad. Sci. USA. 1999;96:742–747. doi: 10.1073/pnas.96.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenfield J. P., Gross R. S., Gouras G. K., Xu H. Front. Biosci. 2000;5:D72–83. doi: 10.2741/greenfield. [DOI] [PubMed] [Google Scholar]
- 3.Greenfield J. P., Leung L. W., Cai D., Kaasik K., Gross R. S., Rodriguez-Boulan E., Greengard P., Xu H. J. Biol. Chem. 2002;277:12128–12136. doi: 10.1074/jbc.M110009200. [DOI] [PubMed] [Google Scholar]
- 4.Caporaso G. L., Takei K., Gandy S. E., Matteoli M., Mundigl O., Greengard P., De Camilli P. J. Neurosci. 1994;14:3122–3138. doi: 10.1523/JNEUROSCI.14-05-03122.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordstedt C., Caporaso G. L., Thyberg J., Gandy S. E., Greengard P. J. Biol. Chem. 1993;268:608–612. [PubMed] [Google Scholar]
- 6.Cai D., Leem J. Y., Greenfield J. P., Wang P., Kim B. S., Wang R., Lopes K. O., Kim S. H., Zheng H., Greengard P., et al. J. Biol. Chem. 2003;278:3446–3454. doi: 10.1074/jbc.M209065200. [DOI] [PubMed] [Google Scholar]
- 7.Rothman J. E. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney D. A., Siddhanta A., Shields D. J. Biol. Chem. 2002;277:3030–3039. doi: 10.1074/jbc.M104639200. [DOI] [PubMed] [Google Scholar]
- 9.Exton J. H. FEBS Lett. 2002;531:58–61. doi: 10.1016/s0014-5793(02)03405-1. [DOI] [PubMed] [Google Scholar]
- 10.Shields D., Arvan P. Curr. Opin. Cell Biol. 1999;11:489–494. doi: 10.1016/s0955-0674(99)80070-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y. G., Siddhanta A., Austin C. D., Hammond S. M., Sung T. C., Frohman M. A., Morris A. J., Shields D. J. Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen Y., Xu L., Foster D. A. Mol. Cell. Biol. 2001;21:595–602. doi: 10.1128/MCB.21.2.595-602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humeau Y., Vitale N., Chasserot-Golaz S., Dupont J. L., Du G., Frohman M. A., Bader M. F., Poulain B. Proc. Natl. Acad. Sci. USA. 2001;98:15300–15305. doi: 10.1073/pnas.261358698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cockcroft S. Cell. Mol. Life Sci. 2001;58:1674–1687. doi: 10.1007/PL00000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai D., Netzer W. J., Zhong M., Lin Y., Du G., Frohman M., Foster D. A., Sisodia S. S., Xu H., Gorelick F. S., et al. Proc. Natl. Acad. Sci. USA. 2006;103:1941–1946. doi: 10.1073/pnas.0510708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddhanta A., Backer J. M., Shields D. J. Biol. Chem. 2000;275:12023–12031. doi: 10.1074/jbc.275.16.12023. [DOI] [PubMed] [Google Scholar]
- 17.Sung T. C., Roper R. L., Zhang Y., Rudge S. A., Temel R., Hammond S. M., Morris A. J., Moss B., Engebrecht J., Frohman M. A. EMBO J. 1997;16:4519–4530. doi: 10.1093/emboj/16.15.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tollefsen S. E., Kornfeld R. J. Biol. Chem. 1983;258:5172–5176. [PubMed] [Google Scholar]
- 19.Annaert W., De Strooper B. Annu. Rev. Cell Dev. Biol. 2002;18:25–51. doi: 10.1146/annurev.cellbio.18.020402.142302. [DOI] [PubMed] [Google Scholar]
- 20.Schwab M. E. Curr. Opin. Neurol. Neurosurg. 1993;6:549–553. [PubMed] [Google Scholar]
- 21.Martin T. F. Annu. Rev. Cell Dev. Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- 22.Powner D. J., Wakelam M. J. FEBS Lett. 2002;531:62–64. doi: 10.1016/s0014-5793(02)03410-5. [DOI] [PubMed] [Google Scholar]
- 23.Li H. L., Roch J. M., Sundsmo M., Otero D., Sisodia S., Thomas R., Saitoh T. J. Neurobiol. 1997;32:469–480. [PubMed] [Google Scholar]
- 24.Watanabe H., Yokozeki T., Yamazaki M., Miyazaki H., Sasaki T., Maehama T., Itoh K., Frohman M. A., Kanaho Y. J. Biol. Chem. 2004;279:37870–37877. doi: 10.1074/jbc.M402610200. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe H., Yamazaki M., Miyazaki H., Arikawa C., Itoh K., Sasaki T., Maehama T., Frohman M. A., Kanaho Y. J. Neurochem. 2004;89:142–151. doi: 10.1111/j.1471-4159.2004.02308.x. [DOI] [PubMed] [Google Scholar]
- 26.McDermott M., Wakelam M. J., Morris A. J. Biochem. Cell Biol. 2004;82:225–253. doi: 10.1139/o03-079. [DOI] [PubMed] [Google Scholar]
- 27.Cowburn R. F., O’Neill C., Bonkale W. L., Ohm T. G., Fastbom J. Biochem. Soc. Symp. 2001;67:163–175. doi: 10.1042/bss0670163. [DOI] [PubMed] [Google Scholar]
- 28.Lee H. C., Fellenz-Maloney M. P., Liscovitch M., Blusztajn J. K. Proc. Natl. Acad. Sci. USA. 1993;90:10086–10090. doi: 10.1073/pnas.90.21.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanfer J. N., Hattori H., Orihel D. Ann. Neurol. 1986;20:265–267. doi: 10.1002/ana.410200214. [DOI] [PubMed] [Google Scholar]
- 30.Borchelt D. R., Thinakaran G., Eckman C. B., Lee M. K., Davenport F., Ratovitsky T., Prada C. M., Kim G., Seekins S., Yager D., et al. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 31.Xu H., Shields D. J. Cell Biol. 1993;122:1169–1184. doi: 10.1083/jcb.122.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berechid B. E., Thinakaran G., Wong P. C., Sisodia S. S., Nye J. S. Curr. Biol. 1999;9:1493–1496. doi: 10.1016/s0960-9822(00)80121-9. [DOI] [PubMed] [Google Scholar]
- 33.Wong P. C., Zheng H., Chen H., Becher M. W., Sirinathsinghji D. J., Trumbauer M. E., Chen H. Y., Price D. L., Van der Ploeg L. H., Sisodia S. S. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 34.Cai D., Shen Y., De Bellard M., Tang S., Filbin M. T. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 35.Leem J. Y., Vijayan S., Han P., Cai D., Machura M., Lopes K. O., Veselits M. L., Xu H., Thinakaran G. J. Biol. Chem. 2002;277:19236–19240. doi: 10.1074/jbc.C200148200. [DOI] [PubMed] [Google Scholar]
- 36.Freyberg Z., Sweeney D., Siddhanta A., Bourgoin S., Frohman M., Shields D. Mol. Biol. Cell. 2001;12:943–955. doi: 10.1091/mbc.12.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musch A., Xu H., Shields D., Rodriguez-Boulan E. J. Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H., Gouras G. K., Greenfield J. P., Vincent B., Naslund J., Mazzarelli L., Fried G., Jovanovic J. N., Seeger M., Relkin N., et al. Nat. Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.