Abstract
Plasmodium vivax uses a single member of the Duffy binding-like (DBL) receptor family to invade erythrocytes and is not found in West Africa where its erythrocyte ligand, the Duffy blood group antigen, is missing. In contrast, Plasmodium falciparum expresses four members of the DBL family, and remarkably, single-point mutations of two of these receptors (BAEBL and JESEBL) bind to entirely different erythrocyte ligands, greatly expanding the range of erythrocytes that P. falciparum can invade. In this article, we describe the molecular basis of the binding specificity for one BAEBL variant (VSTK) that binds to glycophorin C. We demonstrate that soluble glycophorin C completely blocks the binding of BAEBL (VSTK) to human erythrocytes, requiring 0.7 μM for 50% inhibition, a concentration similar to that required by glycophorin A to block the binding of erythrocyte-binding antigen 175 to erythrocytes. BAEBL (VSTK) does not bind to Gerbich-negative erythrocytes that express a truncated form of glycophorin C because it lacks exon 3. The N-linked oligosaccharide of Gerbich-negative glycophorin C has a markedly different composition than the wild-type glycophorin C. Moreover, removal of the N-linked oligosaccharide from the wild-type glycophorin C eliminates its ability to inhibit binding of BAEBL (VSTK) to erythrocytes. These findings are consistent with the ligand for BAEBL (VSTK) being, in part, the N-linked oligosaccharide and suggest that single-point mutations in BAEBL allow P. falciparum to recognize oligosaccharides on different erythrocyte surface glycoproteins or glycolipids, greatly increasing its invasion range.
Keywords: mutations, oligosaccharides, DBL family
Redundancy in erythrocyte invasion pathways is a critical factor in the survival of Plasmodium falciparum. Unlike Plasmodium vivax, which was eliminated from West Africa because of the absence of its erythrocyte ligand, the Duffy blood group antigen (1), there are no known mutations in erythrocyte surface proteins that lead to refractoriness to erythrocyte invasion by P. falciparum. The protein on the surface of P. vivax that binds the Duffy blood group antigen belongs to a family of genes in Plasmodium called the Duffy binding-like (DBL) family (2). P. falciparum expresses four DBL genes compared with a single gene in P. vivax, greatly expanding the potential receptor-ligand interactions for P. falciparum (2, 3). In addition, P. falciparum is able to recognize different erythrocyte ligands through single-point mutations in the receptor domain (region II) in two of its four DBL genes (BAEBL and JESEBL) (4, 5), further ensuring survival. Each clone of BAEBL is identified by four polymorphic amino acids in region II (4). One of the variants, BAEBL (VSTK), binds glycophorin C based on its inability to bind Gerbich-negative erythrocytes that lack exon 3 in glycophorin C (4, 6, 7). Because point mutations in BAEBL region II affect the receptor specificity for its ligand on erythrocytes, it is critical to determine the ligand recognized by BAEBL.
Is it likely that the BAEBL variants that are so similar in sequence recognize different peptide backbones as their erythrocyte ligands? It is our view that point mutations in BAEBL are more likely to recognize oligosaccharides than peptides on glycophorin C or on other proteins. Indeed, the extracellular portion of glycophorin C has no homology with any other protein in the human database. Furthermore, treatment of erythrocytes with trypsin, proteinase K, or pronase could not abolish the binding of BAEBL (INKK) to these enzyme-treated erythrocytes (8), suggesting that the ligand is either a glycolipid or a highly protease resistant glycoprotein. Thus, a more viable alternative to a peptide ligand is the possibility that the N-linked oligosaccharide either alone or in combination with O-linked oligosaccharides provides the basis for the diversity of erythrocyte ligands recognized by the BAEBL variants.
We studied the inhibition of binding of the BAEBL variant that fails to bind Gerbich-negative erythrocytes and compared it with those that bind Gerbich-negative erythrocytes using purified intact glycophorin C. We found that the only BAEBL variant that could not bind Gerbich-negative erythrocytes was inhibited by intact glycophorin C. Removal of the N-linked oligosaccharide from glycophorin C abolished the inhibition. Furthermore, we demonstrate that the N-linked oligosaccharide on glycophorin C from Gerbich-negative erythrocytes is different in composition compared with the N-linked oligosaccharide on wild-type glycophorin C. These findings provide evidence that the ligand for BAEBL is an N-linked oligosaccharide or a cluster of N- and O-linked oligosaccharides and have important implications for the mechanism by which BAEBL mutations are selected and for the survival of P. falciparum.
Results and Discussion
We previously demonstrated that four of the variants of BAEBL, each differing in a single amino acid in region II, displayed a different specificity for erythrocytes as determined by their binding patterns to trypsin- and neuraminidase-treated erythrocytes and to Gerbich-negative erythrocytes (4, 6). The region II domain of BAEBL that binds erythrocytes has mutations in only four amino acids in 20 clones of P. falciparum sequenced from around the world (4). The four amino acids at these positions define five BAEBL variants (VSTK, VSKK, ISKK, INKK, and INRE). All BAEBL variants with the exception of INKK were studied (4), and only the VSTK variant failed to bind to Gerbich-negative erythrocytes, which expresses a truncated form of glycophorin C, indicating that glycophorin C is the erythrocyte ligand for the VSTK variant (4).
Binding of BAEBL (VSTK) to Erythrocytes Is Inhibited by Glycophorin C.
To further analyze the specificity of the BAEBL variants for glycophorin C, we determined the ability of different concentrations of soluble glycophorin C to inhibit binding of four of the five BAEBL variants to human erythrocytes. To study the effect of soluble glycophorin C on the binding of BAEBL (VSTK) to human erythrocytes, the supernatant from P. falciparum Dd2(NM)-infected erythrocytes expressing the BAEBL (VSTK) was first incubated with purified glycophorin C at a concentration range from 0.3 to 100 μM before adding the mixture to erythrocytes. The inhibition, or lack thereof, was determined by the quantity of parasite protein bound to erythrocytes. We found purified glycophorin C completely blocked the binding of the BAEBL (VSTK) to erythrocytes at concentrations of 20–30 μM and that 50% inhibition was achieved at a concentration of 0.7 μM (Fig. 1). Notably, another member of the DBL family, erythrocyte-binding antigen 175, fails to bind to human erythrocytes in the presence of soluble glycophorin A in the same concentration range (9).
Fig. 1.
Glycophorin C completely inhibits the binding of BAEBL (VSTK) but not other BAEBL variants to human erythrocytes. (A) Immunoprecipitation of BAEBL variants VSTK (Dd2Nm), ISKK (E12), INKK (3D7), and INRE (MCamp) eluted from human erythrocytes following incubation with various concentrations of glycophorin C. (B) The percent inhibition of binding of VSTK by different concentrations of glycophorin C BAEBL to human erythrocytes was obtained separately three times; the inhibition of binding of the other variants by glycophorin C was performed twice. Data are shown as the mean of these experiments, and the error bars are the standard deviation. The intensity of the BAEBL band was measured by using imagej (http://rsb.info.nih.gov/ij/), and the data are expressed as the percentage of binding of BAEBL to erythrocytes that were not preincubated with glycophorin C. The inhibition of binding of VSTK BAEBL reached 50% at 0.7 μM.
Three of the BAEBL variants (INRE, ISKK, and INKK) lacked specificity for glycophorin C, showing no inhibition of binding by glycophorin C even at a concentration of 100 μM (Fig. 1). These data demonstrate that glycophorin C is the erythrocyte ligand for only the VSTK variant of BAEBL and is not the ligand for the other three highly related variants.
Contrary to our results, Lobo et al. (10) concluded that glycophorin C was the ligand for BAEBL variant (INKK) based on the observation that INKK did not bind to Leach erythrocytes that lack glycophorin C or to Yus erythrocytes that have a deletion of exon 2 of glycophorin C. It was also observed in the previous study that antibodies to glycophorin C partially inhibited the binding of INKK to erythrocytes (10). However, these results are not entirely conclusive because binding to Leach and Yus erythrocytes was performed with erythrocytes frozen as pellets in liquid nitrogen that may have been unsuitable for the binding assay. In addition, an alternative explanation for the partial inhibition by antibody to glycophorin C is that the antibodies affected the function of another protein, perhaps through band 4.1, which connects glycophorin C to the cytoskeleton (11). For example, band 4.1 also interacts with band 3 and CD44 (12). Furthermore, there is evidence that band 3 is one of the ligands for parasite invasion (13). Our observation that the binding of BAEBL (INKK) to erythrocytes could not be inhibited by purified, soluble glycophorin C at a concentration 100-fold greater than that required for blocking of binding of VSTK to erythrocytes strongly suggests that glycophorin C is not the ligand for BAEBL (INKK).
Glycophorin A Fails to Inhibit Binding of BAEBL to Erythrocytes.
It has been reported that glycophorin A, in addition to glycophorin C, could act as the ligand for BAEBL (VSTK) and BAEBL (INKK) (7). This suggestion was based on the binding of BAEBLs (VSTK and INKK) in a Western overlay assay to the region of the gel where the dimer of glycophorin A migrates. In contrast, we found that 100 μM of glycophorin A had no effect on the binding of VSTK, VSKK, ISKK, or INRE to erythrocytes (data not shown).
Cleavage of the N-Glycan from Glycophorin C Destroys Its Ability to Inhibit Binding of BAEBL (VSTK) to Erythrocytes.
What is the biochemical nature of the ligand on glycophorin C that BAEBL (VSTK) recognizes? As discussed above, we hypothesized that the ligand may be the N-linked oligosaccharide alone or in combination with O-linked oligosaccharides. To determine whether the N-linked oligosaccharide on glycophorin C was critical for binding to BAEBL (VSTK), glycophorin C was treated with protease-free N-glycanase under nondenaturing conditions, and the products were recovered by size-exclusion chromatography on Sephadex G-50 columns (Fig. 2). Carbohydrate compositional analysis showed that although untreated glycophorin C had relatively high levels of mannose (Man) and GlcNAc, the N-glycanase-treated glycophorin C completely lacked Man and had only a low level of GlcNAc (Table 1), indicating complete removal of the N-linked oligosaccharide. In contrast, GalNAc quantitatively remained associated with the N-glycanase-treated glycophorin C, demonstrating that the enzyme did not remove the O-linked glycans.
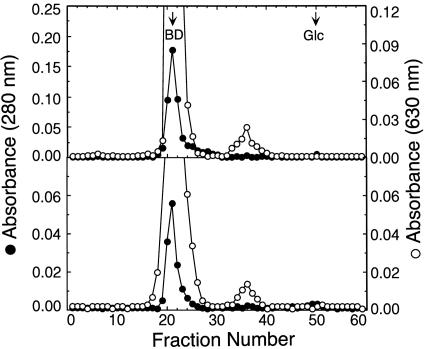
Fig. 2.
Preparation of N-deglycosylated glycophorin C of N-linked glycans for carbohydrate analysis (Table 1). Normal (Upper) and Gerbich-negative glycophorin C (Lower) were N-deglycosylated with N-glycanase and chromatographed on Sephadex G-50 columns. Fractions having the released N-glycans (fraction numbers 32–41) were combined and dialyzed by using membranes with a 500-Da molecular mass cut-off and lyophilized. The void volume determined by chromatographing blue dextran (BD) and the elution volume of glucose (Glc) are indicated by arrows.
Table 1.
Carbohydrate composition of normal and Gerbich-negative glycophorin C before and after N-deglycosylation
| Sample | Sugar composition (relative molar ratios)* |
||||
|---|---|---|---|---|---|
| GalNAc | GlcNAc | Gal | Man | NeuNAc | |
| Normal glycophorin C | 11.4 | 6.0 | 13.1 | 3.0 | 20.5 |
| N-deglycosylated normal glycophorin C | 11.8 | 1.7 | 12.1 | ND | 18.2 |
| N-linked glycans of normal glycophorin C | ND | 4.8 | 1.9 | 3.0 | 1.8 |
| Gerbich-negative glycophorin C | 4.8 | 2.9 | 5.8 | 8.0 | 6.7 |
| N-deglycosylated Gerbich-negative glycophorin C | 5.0 | 0.2 | 4.9 | ND | 5.6 |
| N-linked glycans of Gerbich negative glycophorin C | ND | 2.5 | 1.1 | 7.8 | 0.9 |
Untreated glycophorin C, N-deglycosylated glycophorin C, and N-linked glycans released by treatment of glycophorin C with N-glycanase were hydrolyzed with either 2.5 M trifluoroacetic acid (to release neutral sugars and hexosamines) or 2 M acetic acid (to release sialic acid). The hydrolysates were dried, and the sugars were analyzed by high-pH anion-exchange HPLC. ND, not detected.
*The sugars in the hydrolysates were quantitated from the chromagraphic peak areas and by using standard sugar solutions.
Furthermore, immunoblotting indicated that although untreated glycophorin C electrophoresed as a diffuse band with an average molecular mass of 36.5 kDa, the N-glycanase-treated glycophorin C migrated as a somewhat less diffuse band with a molecular mass of 33.7 kDa on SDS/PAGE (Fig. 3A). This result shows that there was no cleavage of glycophorin C due to a protease contaminant in the N-glycanase. In the case of Gerbich-negative glycophorin C, the mobility of the untreated and N-glycanase-treated glycoprotein corresponded to 31.9 and 29.4 kDa, respectively.
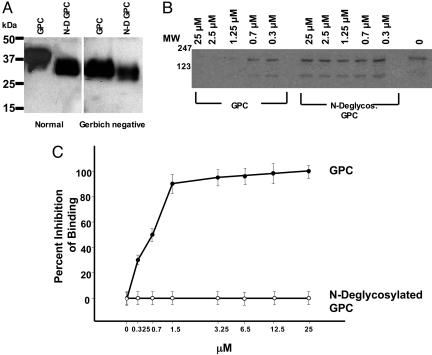
Fig. 3.
N-deglycosylated glycophorin C did not inhibit binding of BAEBL (VSTK) to human erythrocytes. (A) Untreated (GPC) and N-glycanase-treated (N-D GPC) normal glycophorin C and Gerbich-negative glycophorin C were analyzed by Western blotting by using anti-glycophorin C antibody. The positions of molecular mass (kDa) marker proteins are indicated. (B) Immunoprecipitation of BAEBL (VSTK) eluted from human erythrocytes preincubated with either glycophorin C (GPC) or N-deglycosylated glycophorin C (N-Deglycos. GPC). The molecular weight standards are shown on the left. (C) The percent inhibition of binding of VSTK BAEBL to erythrocytes by glycophorin C and N-deglycosylated glycophorin C. Data are shown as the mean of two independent experiments, and the error bar is the standard deviation.
The effect of N-deglycosylated glycophorin C on the binding of BAEBL (VSTK) to erythrocytes was determined. The N-deglycosylated glycophorin C was unable to inhibit the binding of VSTK BAEBL to erythrocytes at a concentration ≤25 μM, whereas intact glycophorin C tested in parallel completely inhibited the binding in a dose-dependent manner, as described above (see Fig. 3 B and C). Nevertheless, the N-glycans released from glycophorin C were unable to inhibit the binding of glycophorin C to a concentration ≤100 μM (data not shown). Taken together, these data demonstrate that the N-linked oligosaccharide on glycophorin C was necessary for the binding of BAEBL (VSTK) to glycophorin C, but the isolated N-glycans themselves were not sufficient to inhibit the binding.
The Biochemical Nature of the N-Glycan on Gerbich-Negative Glycophorin C.
The above observations raise the question of whether the N-linked oligosaccharide on Gerbich-negative glycophorin C differs from the wild-type glycophorin C. Indeed, we found that the N-glycan on Gerbich-negative and normal glycophorin C differed in their composition (Table 1). The N-linked oligosaccharide from erythrocytes with normal glycophorin C consists of NeuNAc/Gal/GlcNAc/Man in the molar ratios of 1.8:1.9:4.8:3.0 (Table 1). In contrast, the N-linked oligosaccharide of Gerbich-negative erythrocytes contained a markedly elevated proportion of Man (Table 1), suggesting the presence of a considerable amount of high Man-type structures in addition to sialylated complex-type oligosaccharides. Thus, glycophorin C of Gerbich-negative erythrocytes differed not only by a deletion in exon 3, leading to a truncated protein, but also in the carbohydrate composition of its N-linked oligosaccharide. This difference in the N-linked oligosaccharide and the failure to inhibit erythrocyte binding with N-linked deglycosylated glycophorin C is consistent with the N-linked oligosaccharide being the ligand for the BAEBL (VSTK) variant. N-glycans encompass a diverse repertoire of polymeric branched structures initially added to proteins as high Man structures in the endoplasmic reticulum and subsequently processed to a variety of complex structures in the Golgi apparatus (14). Evidence has been presented from the Robbins Laboratory (Massachusetts Institute of Technology, Cambridge, MA) that physical accessibility of the oligosaccharide affects its processing in the Golgi (15).
The O-Linked Oligosaccharides on Gerbich-Negative Glycophorin C.
It was found that Gerbich-negative glycophorin C had reduced O-linked oligosaccharides compared with wild-type glycophorin C (Table 1). The exon-3 deletion of Gerbich-negative glycophorin C does not eliminate any serines or threonines on the protein to which O-linked oligosaccharides are linked. The reduced mol of O-linked oligosaccharides may result from the diminished access of the shortened protein to the glycosyltransferases in the Golgi, similar to the explanation for the high Man structure of the N-linked oligosaccharide.
Concluding Remarks.
A question raised by our findings is why the purified N-linked oligosaccharide itself could not inhibit binding. This result is consistent with the finding that glycophorin A, which has a similar N-linked oligosaccharide content (16) to glycophorin C, did not inhibit binding of BAEBL (VSTK) to erythrocytes. One possibility is that the sialic acid on the oligosaccharide interacts with positively charged amino acid on the peptide backbone in a way that exposes the ligand. It is also possible that clusters of sialic acid residues of N-linked oligosaccharides and those of several closely spaced O-linked oligosaccharide in glycophorin C or a conformational structure involving residues of both N- and O-linked oligosaccharides provide multivalency for efficient binding of BAEBL (VSTK).
Is there precedent for a single-point mutation in a receptor altering the receptor for oligosaccharide ligands? A single mutation in the receptor pocket of the hemagglutinin of the influenza virus changes the receptor to allow recognition of α-2,6-sialic acid instead of α-2,3-sialic acid (17). Carbohydrates are extremely versatile in structure and composition (18). They are the ligands of choice for both prokaryotic microorganisms and P. falciparum (19). Variations in the terminal sequences of oligosaccharides, the linkages of these sequences, as well as unusual modifications found in carbohydrates play important biological roles. Such modifications can mask the recognition of many microorganism receptors (20). It has been suggested that the diversity of surface glycan molecules represent a mechanism of pathogen evasion by long-lived vertebrates such as humans (20). Therefore, microorganisms like the influenza virus and P. falciparum may have evolved polymorphisms generated by single-point mutations in their receptors to use the diversity in carbohydrate ligands.
In contrast to BAEBL, point mutations in the P. vivax Duffy-binding receptor does not lead to invasion by a Duffy blood group independent pathway (21). Because of this requirement, P. vivax is not found in West Africa where most people are Duffy blood group-negative (1). The Duffy-binding receptor of P. vivax binds a peptide on the Duffy blood group proteins, although it also binds to a sulfate group on the peptide with higher affinity (22, 23). Thus, it is more likely that single-point mutations in the erythrocyte-binding domain of BAEBL that bind different glycoproteins recognize as their ligand the variation in the distribution and carbohydrate composition of the N- and O-linked oligosaccharides.
Materials and Methods
P. falciparum Clones Used.
The parasite clones used in this study were E12 (ISSK), HB3 (VSTK), Malayan Camp (INRE), 3D7 (INKK), and Dd2/Nm (VSTK). Genotypes of the parasite clones were confirmed by microsatellite analysis (24). Parasite culture and DNA isolation were performed as described in ref. 6.
Erythrocyte-Binding Assay.
Soluble,35S-labeled parasite proteins were obtained from the culture supernatant of each clone as described in ref. 6. 35S-labeled supernatant (100 μl) was used in absorption to erythrocytes and their elution, as described in ref. 6 with the following modifications. The supernatant was incubated with purified glycophorin C in concentrations ranging from 0.3 to 100 μM for 1 h at room temperature with rotation before adding an equal volume of erythrocytes. After a 30-min incubation at room temperature, the mixture was centrifuged at 14,000 × g, and the supernatant was discarded. Erythrocytes were resuspended in 500 μl of RPMI medium 1640 and rapidly layered onto dibutyl phthalate (Sigma). After the mixture was centrifuged at 14,000 × gfor 1 min in a microfuge, supernatant and oil were removed by aspiration. The Eppendorf tube was turned upside down to remove the residual oil and any remaining supernatant. The erythrocyte pellet was washed twice with 500 μl of RPMI medium 1640, and bound parasite proteins were eluted from the erythrocytes with 1.5 M NaCl. The eluate was handled as described in ref. 6, including the immunoprecipitation of BAEBL.
Isolation of Total Glycophorin from Erythrocyte Membranes.
The preparation of erythrocyte membranes and isolation of total glycophorin from the membranes were done as reported in ref. 25. Briefly, ≈400 ml of human blood obtained from a blood bank was washed with cold isotonic buffer and lysed in 4 liters of hypotonic buffer. The membranes were recovered by centrifugation and then extracted with 10 mM Tris·HCl, pH 7.4, containing 85 mM deoxycholate as described in ref. 26. To the extract, an equal volume of 50% phenol was added, stirred, and centrifuged at 5,200 × g. The upper aqueous layer containing total glycophorin was collected, dialyzed against water, and lyophilized. The solid mass was suspended in cold 90% ethanol and stirred to remove the residual phenol and recovered by centrifugation. The pellet of pure glycophorin C was dissolved in water, dialyzed, and lyophilized.
Purification of Glycophorin C.
Glycophorin C was purified from total glycophorin as described in ref. 27. The total glycophorin, obtained from the procedure above, was dissolved in 25 mM phosphate buffer, pH 7.8, containing 25 mM NaCl/0.1% deoxy cholate. The solution was chromatographed on Bio-Gel A 1.5 (2.5 × 90 cm) in the above buffer. Fractions (4.5 ml) were collected, absorption at 260 and 280 mM was measured, and aliquots were analyzed for sialic acid by the periodate–resorcinol method (28). Fractions containing glycophorin C were separately pooled, dialyzed, and lyophilized.
Deglycosylation of Glycophorin C by N-Glycanase.
N-deglycosylation of glycophorin C was performed by using protease-free PNGase F (N-glycosidase F; specific activity of 1,800,000 units/mg; New England Biolabs) purified from Flavobacterium meningosepticum as described in ref. 29. To a solution of glycophorin C (≈2 mg) in 50 mM sodium phosphate, pH 7.5, was added 100 μl of N-glycanase (50,000 units). The reaction mixture was incubated at 37 °C for 18 h and chromatographed on a Sephadex G-50 column (1 × 50 cm) by using PBS, pH 7.2. Fractions (0.66 ml) were collected, and absorption at 280 nm was measured. Aliquots of fractions were analyzed for sialic acid by the microperiodate-resorcinol method (28). Fractions containing glycophorin C and the released N-linked oligosaccharides were pooled separately, dialyzed (the latter by using a 500-Da cut-off dialysis membrane), and lyophilized.
Carbohydrate Composition Analysis.
Untreated glycophorin C, N-deglycosylated glycophorin C, and N-linked oligosaccharides (10 μg of protein or oligosaccharides corresponding to 10 μg of protein) were dissolved in 400 μl of 2.5 M trifluoroacetic acid and hydrolyzed at 100°C for 5 h. The hydrolysates were dried in a Speed-Vac (Savant Instruments, Inc., Farmingdale, NY) and analyzed for neutral sugar and hexosamines on a CarboPac PA10 high-pH anion-exchange column (2 × 250 mm) by using a Dionex BioLC HPLC system coupled to pulsed amperometric detector. Elution was with 20 mM sodium hydroxide. Response factors for the monosaccharides were determined by using standard sugar solutions (30). For sialic acid analysis, samples were dissolved in 2 M acetic acid (300 μl) and hydrolyzed at 80°C for 1 h. The hydrolysates were dried in a Speed-Vac, and the residue was dissolved in water and analyzed as above by using 100 mM NaOH/150 NaOAc as the eluent.
Immunoblot Analysis of Untreated and N-Deglycosylated Glycophorin C.
Untreated or N-deglycosylated glycophorin C (2–3 μg each) was electrophoresed on 4–15% SDS/PAGE in the presence of 2-mercaptoethanol. The protein bands in the gels were electrotransferred onto poly(vinylidene difluoride) membranes, and the membranes were blocked with 5% fat-free dry milk in TBST buffer (50 mM Tris·HCl, pH 7.5, containing 150 mM NaCl/0.05% Tween 20). The membranes were incubated with 1:200-diluted mouse anti-glycophorin monoclonal antibody. The bound antibody was detected with 1:2,000-diluted horseradish peroxidase-conjugated goat anti-mouse IgG and chemiluminescence substrate (Kirkegaard & Perry Laboratories).
Acknowledgments
We thank Dr. Veer P. Bhavanandan for his advice during the preparation of glycophorin C and Dr. Susan Pierce for carefully reviewing the manuscript. Work done in the laboratory of D.C.G. was supported by National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) Grant AI45086. This work was also supported, in part, by the intramural research program of NIAID, NIH.
Glossary
Abbreviations:
- DBL
Duffy-binding ligand
- Man
mannose.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Miller L. H., Mason S. J., Clyde D. F., McGinniss M. H. N. Engl. J. Med. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 2.Adams J. H., Kaneko O., Blair P. L., Peterson D. S. Trends Parasitol. 2001;17:297–299. doi: 10.1016/s1471-4922(01)01948-1. [DOI] [PubMed] [Google Scholar]
- 3.Gaur D., Mayer D. C. G., Miller L. H. Int. J. Parasitol. 2004;34:1413–1429. doi: 10.1016/j.ijpara.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Mayer D. C. G., Mu J. B., Feng X., Su X. Z., Miller L. H. J. Exp. Med. 2002;196:1523–1528. doi: 10.1084/jem.20020750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer D. C. G., Mu J. B., Kaneko O., Duan J., Su X. Y., Miller L. H. Proc. Natl. Acad. Sci. USA. 2004;101:2518–2523. doi: 10.1073/pnas.0307318101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer D. C. G., Kaneko O., Hudson-Taylor D. E., Reid M. E., Miller L. H. Proc. Natl. Acad. Sci. USA. 2001;98:5222–5227. doi: 10.1073/pnas.081075398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maier A. G., Duraisingh M. T., Reeder J. C., Patel S. S., Kazura J. W., Zimmerman J. W., Cowman A. F. Nat. Med. 2003;9:87–92. doi: 10.1038/nm807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson J. K., Triglia T., Reed M. B., Cowman A. F. Mol. Microbiol. 2001;41:47–58. doi: 10.1046/j.1365-2958.2001.02484.x. [DOI] [PubMed] [Google Scholar]
- 9.Sim B. K. L., Chitnis C. E., Wasnioswska K., Hadley T. J., Miller L. H. Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 10.Lobo C. A., Rodriguez M., Reid M., Lustigman S. Blood. 2003;101:4628–4631. doi: 10.1182/blood-2002-10-3076. [DOI] [PubMed] [Google Scholar]
- 11.An X. L., Takakuwa Y., Nunomura W., Manno S., Mohandas N. J. Biol. Chem. 1996;271:33187–33191. doi: 10.1074/jbc.271.52.33187. [DOI] [PubMed] [Google Scholar]
- 12.Nunomura W., Takakuwa Y., Tokimitsu R., Krauss S. W., Kawashima M., Mohandas N. J. Biol. Chem. 1997;272:30322–30328. doi: 10.1074/jbc.272.48.30322. [DOI] [PubMed] [Google Scholar]
- 13.Okoye V. C., Bennett V. Science. 1985;227:169–171. doi: 10.1126/science.3880920. [DOI] [PubMed] [Google Scholar]
- 14.Kornfeld R., Kornfeld S. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh P., Rosner M. R., Robbins P. W. J. Biol. Chem. 1983;258:2555–2561. [PubMed] [Google Scholar]
- 16.Yoshima H., Furthmayr H., Kobata A. J. Biol. Chem. 1980;255:9713–9718. [PubMed] [Google Scholar]
- 17.Rogers G. N., Paulson J. C., Daniels R. S., Shekel J. J., Wilson I. A., Wiley D. C. Nature. 1983;304:76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- 19.Varki A., Cummings R., Esko J., Freeze H., Hart G., Marth J. Essentials of Glycobiology. Woodbury, NY: Cold Spring Harbor Lab. Press; 653. p. 653. [PubMed] [Google Scholar]
- 19.Varki A. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 20.Gagneux P., Varki A. Glycobiology. 1999;9:747–755. doi: 10.1093/glycob/9.8.747. [DOI] [PubMed] [Google Scholar]
- 21.Xainli J., Adams J. H., King C. L. Mol. Biochem. Parasitol. 2000;111:253–260. doi: 10.1016/s0166-6851(00)00315-7. [DOI] [PubMed] [Google Scholar]
- 22.Chitnis C. E., Miller L. H. J. Exp. Med. 1994;180:497–506. doi: 10.1084/jem.180.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hans D., Pattnaik P., Bhattacharyya A., Shakri A. R., Yazdani S. S., Sharma M., Choe H., Farzan M., Chitnis C. E. Mol. Microbiol. 2005;55:1423–1434. doi: 10.1111/j.1365-2958.2005.04484.x. [DOI] [PubMed] [Google Scholar]
- 24.Su X. Z., Carucci D. J., Wellems T. E. Exp. Parasitol. 1998;89:262–265. doi: 10.1006/expr.1998.4299. [DOI] [PubMed] [Google Scholar]
- 25.Zvilichovsky B., Gallop P. M., Blumenfeld O. Biochem. Biophys. Res. Commun. 1971;44:1234–1243. doi: 10.1016/s0006-291x(71)80218-8. [DOI] [PubMed] [Google Scholar]
- 26.Segrest J. P., Wilkinson T. M., Sheng L. Biochim. Biophys. Acta. 1979;554:533–537. doi: 10.1016/0005-2736(79)90389-4. [DOI] [PubMed] [Google Scholar]
- 27.Furthmayr H., Tomita M., Marchesi V. T. Biochem. Biophys. Res. Commun. 1975;65:113–121. doi: 10.1016/s0006-291x(75)80068-4. [DOI] [PubMed] [Google Scholar]
- 28.Jourdain G., Dean L., Roseman S. J. Biol. Chem. 1971;246:430–435. [PubMed] [Google Scholar]
- 29.Maley F., Trimble R. B., Tarentino A. L., Plummer T. H., Jr. Anal. Biochem. 1989;180:195–204. doi: 10.1016/0003-2697(89)90115-2. [DOI] [PubMed] [Google Scholar]
- 30.Hardy M. R. Methods Enzymol. 1989;179:76–82. doi: 10.1016/0076-6879(89)79115-1. [DOI] [PubMed] [Google Scholar]