Abstract
Tissue engineering holds the promise of replacing damaged or diseased tissues and organs. The use of autologous donor cells is often not feasible because of the limited replicative lifespan of cells, particularly those derived from elderly patients. Proliferative arrest can be overcome by the ectopic expression of telomerase via human telomerase reverse transcriptase (hTERT) gene transfection. To study the efficacy and safety of this potentially valuable technology, we used differentiated vascular smooth muscle cells (SMC) and vascular tissue engineering as a model system. Although we previously demonstrated that vessels engineered with telomerase-expressing SMC had improved mechanics over those grown with control cells, it is critical to assess the phenotypic impact of telomerase expression in donor cells, because telomerase up-regulation is observed in >95% of human malignancies. To study the impact of telomerase in tissue engineering, expression of hTERT was retrovirally induced in SMC from eight elderly patients and one young donor. In hTERT SMC, significant lifespan extension beyond that of control was achieved without population doubling time acceleration. Karyotype changes were seen in both control and hTERT SMC but were not clonal nor representative of cancerous change. hTERT cells also failed to show evidence of neoplastic transformation in functional assays of tumorigenicity. In addition, the impact of donor age on cellular behavior, particularly the synthetic capability of SMC, was not affected by hTERT expression. Hence, this tissue engineering model system highlights the impact of donor age on cellular synthetic function that appears to be independent of lifespan extension by hTERT.
Keywords: tumorigenicity, smooth muscle cells, vascular graft
In the emerging field of tissue engineering, a critical consideration is cell source. To create neo-tissues for transplant into a patient, the ideal donor cells would be those that are easily obtainable, readily expandable in vitro without the loss of differentiated phenotype or function, and implantable with little or no immune reaction (1). However, a difficulty in using autologous cells lies in the restricted proliferative capacity of donor cells in vitro. For example, we have recently shown that a critical stumbling block to the clinical application of engineered small caliber vessels using autologous human cells is the limited proliferative lifespan of elderly vascular smooth muscle cells (SMC), which are the primary cellular constituent of engineered vessels and crucial for providing mechanical integrity (2). Vascular SMC that are obtained from elderly patients (those individuals beyond middle age and representing the age group most likely to require coronary revascularization) have an in vitro lifespan as low as 5–10 population doublings. This short lifespan limits their utility for culturing functional blood vessels, because this process ultimately requires 30–40 population doublings (2–4). Vascular SMC and vascular tissue engineering thus represent an excellent model system for studying cellular lifespan with respect to its impact on tissue engineering.
Replicative cellular lifespan is limited by the incomplete replication of the genome during DNA replication, which results in the loss of 50–100 bp from the terminal telomeric repeat sequence with each population doubling. The enzyme telomerase is responsible for the complete replication of telomere ends, but it is primarily active only in prenatal cells or at low levels in adult stem cells. Telomerase activity is primarily carried out by its catalytic subunit, human telomerase reverse transcriptase (hTERT) in humans, which has been cloned. When hTERT is ectopically expressed in normal human cells, the replicative lifespan of these cells is extended through lengthening and stabilization of telomeres.
A number of groups are currently exploring the use of ectopic telomerase expression for tissue engineering and cell therapy applications. Thomas et al. (5) reported on the first use of hTERT expression in xenotransplantion by using bovine adrenocortical cells implanted into severe combined immunodeficient mice to rescue organ function after adrenalectomy. hTERT lifespan extension is also being investigated for the treatment of Duchenne muscular dystrophy by using genetically modified muscle satellite cells (6, 7). Other cell types that have been lifespan-extended by telomerase in anticipation of tissue engineering applications include retinal pigment epithelia (8–12), dermal fibroblasts (13, 14), vascular endothelium (15–17), bone marrow stromal cells (18–21), osteoblasts (22, 23), mesenchymal stem cells (24), and hepatic stellate cells (25, 26). Using retroviral delivery of hTERT to human saphenous vein SMC, it is possible to culture autologous blood vessels for patients as old as 74 years (2).
When considering the utility of cellular lifespan extension for tissue engineering, it is important also to recognize that telomerase plays a role in malignant transformation and is reactivated in up to 97% of human cancers. In light of the potential importance of lifespan extension for human therapeutic applications, coupled with important concerns regarding the safety of retroviral delivery and ectopic expression of telomerase, we undertook a functional study to investigate the safety of hTERT overexpression for tissue engineering. Using vascular smooth muscle cells in a vascular tissue engineering model system, we demonstrate the utility of telomerase overexpression for tissue engineering, and critically examine the tumorigenic potential of those donor cells and the impact of donor age.
Results
SMC from eight elderly donors and from a 17-yo (year-old, yo) donor were infected with hTERT at low population doubling (PD) (at or below passage 4 after explant). Cells were selected with hygromycin, and surviving stable cultures were maintained for further analysis. A population of noninfected SMC from each donor was maintained as a control. hTERT cells from four of the eight donors (57yo, 65yo, 81yo, and 85yo) were used for tumorigenicity studies that are included in this report. Engineered vessels were cultured by using hTERT cells from the remaining four donors (47yo, 55yo, 67yo, and 74yo), as described in ref. 2. In this report, these engineered vessels were analyzed to determine the impact of donor age on vessel characteristics in the setting of hTERT expression.
Telomerase Activity Is Maintained During Prolonged Culture.
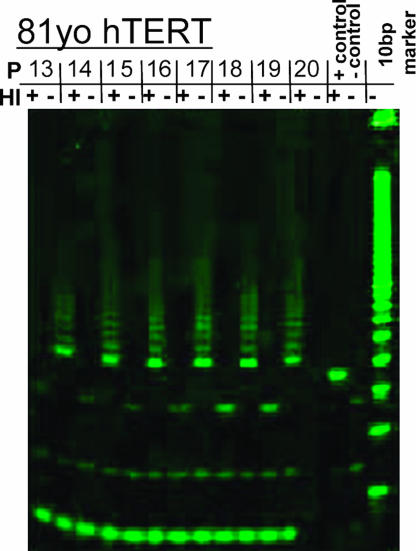
Telomere repeat amplification protocol assay PCRs produced heat-sensitive laddering at 6-bp intervals in positive control and hTERT SMC (typical results for 81yo donor, Fig. 1; see also Fig. 7, which is published as supporting information on the PNAS web site), but not in control populations (data not shown). This observation is consistent with previous results indicating that differentiated saphenous vein SMC do not express endogenous telomerase activity (2). hTERT SMC continued to express telomerase activity in each successive passage for the entirety of passages assayed. 17yo SMC demonstrated telomerase activity up through passage 32, and both the 57yo and 81yo donor SMC continued to show strong telomerase activity for 15–18 passages after infection, when assays were stopped. (Figs. 1 and 7) The 6-bp laddering indicative of telomerase activity showed no diminution at higher passages.
Fig. 1.
Telomere repeat amplification protocol data for representative 81yo hTERT SMC showing characteristic 6-bp laddering indicative of telomerase activity. Telomerase activity persists out to passage 20 (PD 33) after hTERT transfection. HI, heat inactivation, demonstrating that laddering is indicative of telomerase enzyme activity rather than PCR artifact. P, passage number.
Growth Kinetics Similar for hTERT and Control SMC.
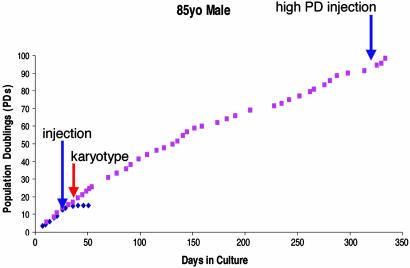
Cancer cells are characterized by uncontrolled proliferation due to the loss of cell cycle checkpoint controls (27). As such, cancer cells often display accelerated growth kinetics with shortened population doubling times (27). We obtained growth curves for control and hTERT SMC populations from each donor. (Typical results are in Fig. 2; see also Fig. 8, which is published as supporting information on the PNAS web site; ref. 2.) Growth in control SMC populations typically plateaued by PD 20 in culture (depending on the donor, Fig. 2 shows results for 85yo patient), whereas hTERT populations continued to divide far beyond the senescence point of control cells. Growth kinetics (measured as average population doubling time on the linear portion of the growth curve) for all cell populations showed significant lengthening of the population doubling time of hTERT over control cells, P = 0.01 by paired test, which would imply that hTERT cells have not undergone tumorigenic transformation. (Table 1) The rate of cell growth, in general, remained stable in hTERT populations beyond the senescence point of control cells, implying continued intact cell cycle regulation in hTERT cells.
Fig. 2.
Representative growth kinetics of hTERT SMC from 85yo donor. Control cells (blue diamonds) reached senescence at <20 PD after transfection. hTERT SMC (red squares) exceeded their control counterparts and continued to proliferate far beyond the point of control senescence. Culture was terminated for hTERT SMC, because they showed no signs of senescence up to 342 days in culture. Red arrows indicate point at which cells were submitted for SKY karyotype analysis. Blue arrows indicate point at which cells were injected into nude mice. 85yo hTERT SMC were also injected into nude mice at high PD.
Table 1.
Growth kinetics of normal and hTERT SMC from elderly donors and 17yo donor
| Vascular cell source | Days in culture | Total PDs | Average PDs/day | Average PD time, days |
|---|---|---|---|---|
| 17yo normal | 84 | 41 | 0.490 | 2.0 |
| 17yo hTERT | 118 | 84 | 0.714 | 1.4 |
| 57yo normal | 61 | 24 | 0.415 | 2.5 |
| 57yo hTERT | 181 | 56 | 0.304 | 3.2 |
| 65yo normal | 51 | 15 | 0.294 | 2.6 |
| 65yo hTERT | 307 | 62 | 0.202 | 4.9 |
| 81yo normal | 22 | 4 | 0.164 | 2.5 |
| 81yo hTERT | 212 | 52 | 0.245 | 2.8 |
| 85yo normal | 51 | 15 | 0.294 | 3.4 |
| 85yo hTERT | 342 | 100 | 0.292 | 3.6 |
All hTERT cultures were arbitrarily stopped as they showed no signs of senescence. Population doubling times for hTERT SMC were significantly slower than corresponding control SMC populations.
Chromosomal Abnormalities Detected in Elderly Cells in Both Control and hTERT Populations.
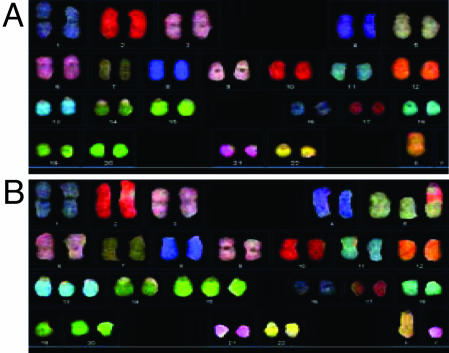
Because certain karyotypic abnormalities are associated with malignancy (28), we performed karyotype analysis of control and hTERT SMC. Timing of karyotype analysis for each donor is shown in Figs. 2 and 8. Spectral karyotype (SKY) analysis of each of four elderly donors revealed aneuploidy in both control and hTERT SMC. (Fig. 3; see also Fig. 9, which is published as supporting information on the PNAS web site). Excluding numerical chromosomal abnormalities which occurred in only one cell and, thus, were nonclonal and likely due to technical errors during cellular preparation, the frequencies of aneuploidy were low for both control and hTERT SMC (2.9–8.2%) (Table 2). Interestingly, the overall rate of karyotypic abnormalities was lower in hTERT populations than in control SMC (Table 2), which is consistent with previous reports of hTERT expression in vascular endothelial cells (15).
Fig. 3.
Representative SKY karyotype images from 85yo hTERT SMC cells. (A) Control SMC showing loss of Y chromosome. (B) hTERT SMC showing monosony 19 and trisomy 5, 13, and 15 but retaining the Y chromosome. Analysis of total cell karyotypes for the 85yo donor revealed the hTERT SMC changes to be technical artifacts; however, the deletion of the Y chromosome in the control SMC represents a true subclone.
Table 2.
Summary of chromosomal abnormalities detected in control and hTERT SMC populations from SKY karyotype analysis
| Donor cell age | Normal |
hTERT |
||
|---|---|---|---|---|
| Abnormality | Frequency | Abnormality | Frequency | |
| 17yo | 47XY, trisomy 5 | 3 of 29 | None | |
| 57yo | 47XY, trisomy 2 | 2 of 67 | 45XO | 4 of 49 |
| 45XY, monosomy 17 | 2 of 67 | |||
| 65yo | 47XY, trisomy 8 | 4 of 69 | None | |
| 45XY, monosomy 18 | 2 of 69 | |||
| 81yo | None | 45XO | 26 of 47 | |
| 46XO, trisomy 15 | 2 of 47 | |||
| 85yo | 45XO | 12 of 46 | None | |
Deletion of the Y chromosome was the most frequently occurring aberration in both normal and hTERT cells and is consistent with aneuploidy seen in cellular aging. The remainder of chromosomal abnormalities occurred at low frequency and are only reported if they represent a true subclone (>2% of cells surveyed).
One notable observation is the 45X, -Y karyotype, which occurred in 26.1% of 85yo control cells and 55.3% of 81yo hTERT cells. Interestingly, in two of the four elderly donors studied (65yo and 85yo), karyotype abnormalities occurred solely in the control cells and not in the hTERT cells. Of the remaining two elderly donors, only the 81yo showed a lack of abnormalities in the control cells and the appearance of abnormalities in hTERT cells. Vascular SMC from a 17yo donor contained numerical abnormalities only in the control cells and not in cells expressing hTERT, similar to what we observed in two of the four elderly donors (Table 2). These data support the concept that chromosomal aberrations occur as a function of donor age and exposure to cell culture conditions and are not increased by ectopic hTERT.
hTERT SMC Are Not Functionally Tumorigenic.
Soft agar assay.
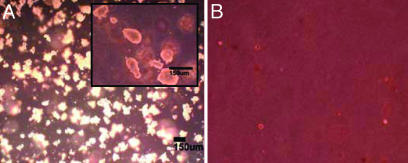
We assayed the ability of hTERT cells to grow in an anchorage-independent manner, a hallmark of cancer cells, by using a clonogenic soft agar assay. Both control and hTERT SMC failed to form any colonies after 3 weeks when grown in soft agar (n = 4, triplicate experiments per donor), whereas control transformed cells (HEK-293) grew multiple colonies (2,870 ± 320). (Fig. 4 showing representative samples and Table 3) Hence, hTERT SMC did not form anchorage-independent tumors in vitro.
Fig. 4.
Soft agar clonogenic assay. (A) HEK-293 cell line used as a positive control and showing formation of numerous colonies after 3 weeks of culture. (Inset) Magnified image of HEK-293 colonies. (B) 81yo donor hTERT SMC showing retention of anchorage dependence. Panels are at the same magnification.
Table 3.
Clonogenic soft agar assay showing retention of anchorage dependence in hTERT SMC
| Cell Type | Mean colony number |
|---|---|
| HEK-293 cells | 2,870 ± 320 |
| Control 17yo SMC | 0 |
| hTERT 17yo SMC | 0 |
| Control 57yo SMC | 0 |
| hTERT 57yo SMC | 0 |
| Control 65yo SMC | 0 |
| hTERT 65yo SMC | 0 |
| Control 81yo SMC | 0 |
| hTERT 81yo SMC | 0 |
| Control 85yo SMC | 0 |
| hTERT 85yo SMC | 0 |
All control and hTERT SMC populations failed to form colonies in soft agar after 3 weeks of culture. Positive control HEK-293 tumor cells produced numerous colonies.
Nude mouse tumorigenicity assay.
The definitive functional assay of tumorigenicity is the in vivo injection of cells into immunodeficient rodents. hTERT SMC from each of the four elderly patients were injected s.c. into female nude mice (time of injection shown in Figs. 2 and 8). After 2 months, no nude mice developed tumors from injected hTERT SMC, whereas control transformed cells (HEK-293) resulted in tumor formation in all mice. (Table 4; see also Fig. 10, which is published as supporting information on the PNAS web site) Because recent studies have shown the ability of hTERT-infected fibroblasts to grow in soft agar at very high passage (29), we also performed injections of high passage (PD 57 for 65yo and PD 95 for 85yo) hTERT SMC from two of the four elderly donors (see Fig. 2). High-passage SMC that were well beyond the point of control cell senescence also failed to form tumors in the nude mice after 2 months, indicating a continued lack of functional tumorigenic transformation (Fig. 10).
Table 4.
In vivo nude mouse tumorigenicity study showing failure of all elderly donor hTERT cells to form tumors in immunodeficient rodents
| Cell type | PD | No. of mice injected | No. of tumors formed |
|---|---|---|---|
| HEK-293 | — | 12 | 12/12 |
| 57yo hTERT | 14 | 2 | 0/2 |
| 65yo hTERT | 4 | 2 | 0/2 |
| 81yo hTERT | 10 | 2 | 0/2 |
| 85yo hTERT | 14 | 2 | 0/2 |
| High PD 65yo hTERT | 57 | 2 | 0/2 |
| High PD 85yo hTERT | 95 | 2 | 0/2 |
hTERT SMC continued to show a failure to form tumors even when injected at high PD far beyond the point of control cell senescence (up to PD 95 in 85yo hTERT SMC). HEK-293 cells, an established tumor cell line, produced tumors in all injected mice and served as a positive control for tumor-forming ability in each mouse.
Vessels Engineered with hTERT SMC.
As we have previously reported, hTERT expression allows for the tissue engineering of robust vascular structures by using SMC derived from elderly donors. Lifespan extension of SMC allowed the growth of engineered vessels in vitro, regardless of donor age (2). Vessels can be engineered from human cells having dimensions that are suitable for implantation without employing stem cells or exceedingly long culture times (typical example in Fig. 5).
Fig. 5.
Example of a tissue-engineered blood vessel. Photograph shows vessel that was engineered from 47yo hTERT SMC. Vessel is ≈5.5 cm in length, 3 mm in internal diameter, and ruptured at 569 mm Hg.
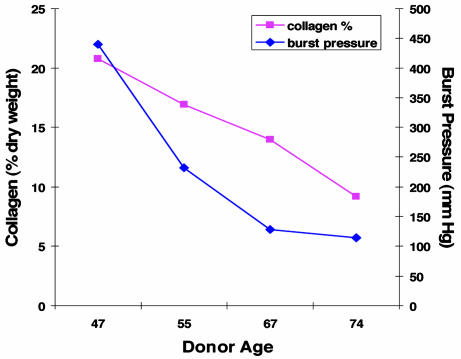
Whereas lifespan extension makes vessel culture from vascular cells feasible, the impact of donor age per se on vessel characteristics has not been well characterized. Hence, we evaluated the impact of donor age on collagen accumulation and mechanical characteristics of vessels engineered from hTERT SMC from four human donors (Fig. 6). Despite extension of cellular lifespan, vessels exhibited decreases in both collagen accumulation (as determined by hydroxyproline content) and burst strength as a function of donor age. This result implies that removing limitations on cellular replication does not render all SMC populations functionally similar. Indeed, age-dependent decrements in matrix synthesis, and consequent decrements in vessel mechanical properties (4), persist despite hTERT gene therapy.
Fig. 6.
Comparison of collagen content and burst pressure in blood vessels engineered from hTERT SMC from four elderly donors. Vessel collagen content (as percent of dry weight) decreases linearly with increasing donor age from 21% in the younger 47yo donor to only 9% in the 74yo donor. Vessel rupture strength also decreases with increasing donor cell age (and decreasing collagen content).
Discussion
Vascular engineering for arterial bypass grafting is a therapeutic modality that is primarily targeted to an aging patient population. Vascular cells, including SMC, derived from these individuals may possess accumulated genetic mutations that could, in the setting of telomerase reactivation, lead to malignant transformation. Hence, vascular SMC from older donors that are used for vascular tissue engineering provide an excellent model system to study the functional impact of telomerase overexpression in tissue engineering. Our previous reports on hTERT in vascular tissue engineering have focused on molecular evaluation of the regulation of cell cycle proteins (2, 30), which could miss low-frequency events that may lead to tumorigenesis from a single cell within a large population. Because of this limitation, in the current study, we paid special attention to the functional evaluation of hTERT SMC and their potential for tumorigenic change, both in vitro and in vivo.
Numerous investigators have shown the feasibility of cellular lifespan extension by using hTERT gene therapy (8, 15, 31–34). Although cellular immortalization in the vast majority of human tumors relies on derepression of the native cellular hTERT gene, telomerase is itself not widely believed to be an oncogene that is independently capable of inducing tumor formation (35). The overexpression of telomerase activity in cultured cells has failed to result in malignant change in a number of cell types, including fibroblasts (12, 33), endothelial cells (15, 33), skeletal muscle cells (36), and esophageal squamous cells (31).
In contrast to these results, several investigators have found that lifespan extension and prolonged cell culture may increase the risk that some cell types can undergo transformation. hTERT immortalization of cells may be permissive for the evolution of particular genetic and/or epigenetic changes that can predispose to malignancy. This phenomenon may depend on duration of culture, cell type, and donor age.
Mondello et al. (29) observed karyotype abnormalities (both numerical and structural) and anchorage-independent growth in soft agar of fibroblasts that were isolated from two centenarian patients and subsequently infected with hTERT by using a retroviral vector. However, no anchorage-independent growth was observed before PD 60, and no hTERT cell populations that were injected into immunodeficient mice produced tumors after 3 months, despite inoculation at PD107 (29). Milyavsky et al. (37) showed that a subpopulation of hTERT-immortalized fibroblasts gradually developed inactivation of p16INK4A and associated loss of contact inhibition as well as increased susceptibility to transformation by H-Ras. Burns et al. (38) describe the tumorigenic transformation of adult human mesenchymal stem cells transfected with hTERT after very prolonged culture (>200 PDs). This transformation was associated with genetic and/or epigenetic changes, including loss of p16INK4a/p14ARF and silencing of the DBCCR1 tumor suppressor gene (38). However, as the authors themselves note, the consequences of hTERT expression are likely different between adult somatic cells and stem cells. Serakinci and colleagues (39) recently demonstrated an example of spontaneous tumorigenicity in telomerized human cells by using adult mesenchymal stem cells. Indeed, it is now postulated that tumors may derive from transformed stem cells, perhaps indicating an intrinsically higher tumorigenic potential in stem cells as opposed to differentiated somatic cells (38).
We used vascular SMC from older donors as a model system for studying the impact of hTERT on cellular function in tissue engineering. As a marker for potential tumorigenicity, we undertook a careful cytogenetic analysis of hTERT SMC by SKY karyotyping. We observed a low frequency of abnormalities that were associated with subclone development in both normal and hTERT SMC. These changes appeared to be sporadic rather than associated with telomerase gene therapy, because in three of the five cell populations (17yo, 65yo, and 85yo) there were subclone abnormalities in the controls but not in the hTERT cells.
The most prevalent abnormality observed, and the one associated with significant subclone numbers, was 45X, -Y (loss of the Y chromosome). Sex chromosomes are the most commonly lost chromosomes associated with aneuploidy in aging cells (40). A search of the Mitelman Database of Chromosome Aberrations in Cancer revealed that in 10 of the 17 documented cases of “blood vessel” tumors, the tumor karyotype was 45X, -Y. However, nonendothelial primary vascular tumors (i.e., leiomyosarcomas derived from SMC) are exceedingly rare. Just over 200 primary venous leiomyosarcomas have been reported in the world literature (41), whereas primary arterial leiomyosarcomata are five times less common than venous tumors (42). Thus, vascular SMC appear to be particularly refractory to tumorigenic change, despite common karyotypic abnormalities.
In light of these findings, and the fact that more control than hTERT SMC exhibited abnormal karyotypes, our observed karyotypic abnormalities are likely due to cell age and/or protracted in vitro culture (11) rather than being causally linked to retroviral telomerase gene therapy or malignant transformation. In fact, telomerase activity may be cytogenetically protective, because it has been shown that tumor initiation in mice is related to genetic instability caused by lack of telomerase (35). Critically short telomeres are subject to recombination events that increase chromosomal instability (43). The results of our karyotypic analysis support this observation. In addition, both in vitro and in vivo assays of tumor formation showed no tumors of either hTERT or control SMC.
The question remains as to whether any potential risk of ex vivo hTERT gene therapy is acceptable within the context of a potentially life-saving tissue engineering therapy. Here we present evidence indicating that hTERT infection of elderly human vascular SMC does not lead to a transformed phenotype at population doublings <100. Although this observation in vascular SMC is somewhat comforting, avoiding constitutive telomerase activity by employing a transient expression of hTERT may be more desirable from a regulatory standpoint. This issue is especially true if other types of tissue engineering are under consideration, particularly those employing mesenchymal stem cells or other precursor cells. Possible strategies for the transient delivery of hTERT into cells include adenoviral vectors or nonviral methods of DNA transfer. However, the short duration of gene expression achieved by these methods may not allow for sufficient telomere elongation to ensure adequate cell replicative potential for tissue engineering applications. In light of this consideration, one additional potential approach is the transient delivery of a highly processive mutant of telomerase that rapidly elongates telomeres in vitro (44).
Irrespective of the method of delivery of hTERT, the issue remains that, although lifespan extension of elderly donor cells facilitates the engineering of tissues from these cells, lifespan extension is not de facto sufficient to ameliorate the impact of cellular aging. In our SMC model system, we observed that with increased donor cell age, collagen synthesis by SMC decreases and consequently reduces the strength of the engineered vessels. This age-related functional change in vascular SMC was not reversed by ectopic hTERT expression, despite rescue of these cells from replicative senescence. Thus, although hTERT expression extends SMC lifespan beyond senescence, it does not reverse biochemical aging of the cells, which is in contrast to some previous reports for vascular endothelial cells (15). Future research should focus on understanding this phenomenon that lifespan extension does not alter changes in cell biology conferred by aging. In addition, improvements in culture strategies and better insights into the regulation of differentiated cellular functions, such as extracellular matrix production, should be the focus of future investigations.
Materials and Methods
Cell Isolation and Culture.
Discarded saphenous vein segments were obtained with Institutional Review Board approval from eight elderly male patients (aged 47–85 years, mean 66 years) who were undergoing coronary artery bypass grafting at Duke University Medical Center. Vascular SMC were harvested by using standard explant techniques as described in ref. 45. Briefly, the saphenous vein was incised longitudinally, and the endothelial cells were removed by vigorously scraping the surface of the lumen with a scalpel blade to expose the medial layer of the vessel. The vessel was then divided into 1-cm sections and placed lumen-side down in 60-mm Petri dishes containing SMC medium [DMEM (GIBCO) supplemented with 20% FBS (HyClone) and 1% penicillin/streptomycin/bFGF/insulin/EGF (all from Sigma)] and cultured at 37°C and 10% CO2 for 2 weeks to allow for SMC migration from the vessel onto the dish. Human aortic SMC from a 17yo donor were purchased (Cambrex) to serve as an age control for comparison with the elderly donor vascular cells.
hTERT Gene Transfer.
The pBABE-Hygro-hTERT plasmid, containing the hTERT gene and a hygromycin resistance genes for selection, was obtained as a kind gift from Christopher Counter (Duke University) (9). The plasmid was packaged into retrovirus by using the pCL-10A1 helper plasmid (Imgenex, San Diego) and HEK-293 cells. The resultant medium contained amphotrophic retrovirus carrying the hTERT gene. A subculture of SMC from each patient at passage 2 or 3 and 70–80% confluence was exposed to retrovirus-containing medium supplemented with 8 μg/ml Polybrene (Sigma) for 12 h at 37°C. Forty-eight hours after infection, the infected-cell populations were selected and maintained in culture medium containing 60 μg/ml hygromycin to ensure a stably infected population. A population of noninfected cells from each patient was maintained in parallel as a control.
Telomeric Repeat Amplification Protocol Assay.
Telomerase activity, in cells infected with retrovirus containing the hTERT cDNA, was detected by using the commercial TRAPeze Telomerase Detection Kit (Chemicon) according to manufacturer's protocol. Briefly, cells were lysed by using 1× 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate lysis buffer, and the resulting supernatant was quantified by Bradford Assay. PCRs were set up by using 500 ng of cell extract protein for 30 cycles. Amplified samples were run on 8% PAGE and stained with SYBR Green I (Molecular Probes) to identify telomerase activity.
Growth Kinetics.
Growth kinetics were determined for control and hTERT cells by serial passage at subconfluence. Cell culture was continued until cells reached senescence or for a maximum of 342 days, whichever came first. Cells were counted with each passage to determine the number of PD. PDs were plotted against time, and the average PD time was calculated from the linear growth phase for both control and hTERT cells from each patient.
Karyotype Analysis.
For chromosomal studies, actively proliferating cultures of normal and hTERT cells were analyzed by spectral karyotyping (Roswell Cancer Institute, Buffalo, NY; SKY Analysis). The mitotic inhibitor nocodazole (Calbiochem) was used to induce cell-cycle arrest. Chromosome spreads were prepared by using air-drying methods. After sequential digestion with RNase and pepsin, chromosomal DNA was denatured in 70% formamide and then hybridized with a mixture of human SKY paint probes that were tagged with various nucleotide analogues, as described in refs. 46–49. Thirty to fifty mitoses were chosen at random, and their images, developed by combinations of five different flouorophores, were captured with a spectral cube and interferometer module installed on a Nikon microscope. Spectral karyotypes were captured by using sky view 1.62 software.
Clonogenic Soft Agar Assay for Anchorage Independent Growth.
To determine functional tumorigenicity in vitro, hTERT and control SMC from each patient were suspended at 1 × 105 cells/ml in culture medium containing 0.36% agar (Sigma) as described in ref. 50. This cell suspension was added to six-well plates that were precoated with 2 ml of 0.9% solid agar. Plating was done in duplicate. Colonies were manually counted after 3 weeks of culture. HEK-293 cells, which express T/t antigen, hTERT, and mutant Ras, were used as a positive control.
Nude Mouse Tumorigenicity Assay.
To determine functional tumorigenicity in vivo, hTERT SMC were suspended in DMEM (GIBCO) and inoculated s.c. into one flank of 4-week-old female nude mice (nu/nu; Harlan, Indianapolis) at a concentration of 3 × 106 cells in 0.3 ml. As a positive control, HEK-293 cells at the same concentration were injected s.c. into the opposite flank of each mouse. Mice were injected in duplicate and monitored for up to 2 months to follow tumor growth. At the end of this period, or when tumor mass exceeded a volume of 1 cm3, animals were killed, and tumor tissue was excised and formalin fixed for hematoxylin/eosin staining.
Culture of Engineered Vessels.
Blood vessels were cultured under biomimetic pulsatile conditions, as described in ref. 4. Control and hTERT SMC were separately seeded at passage 6 or below onto tubular polyglycolic acid scaffolds that were suspended within bioreactors over compliant silicone tubing. Bioreactors were filled with SMC medium and incubated at 37°C and 10% CO2. Pulsatile flow through the lumen of the vessel construct was begun on day 5 of culture at a rate of 165 beats per minute to produce ≈1% radial distension. Vessels were cultured for a total of 7–8 weeks. Mechanical testing of mature vessel segments was performed, as previously described, to determine vessel burst strength and mechanical properties (4). Collagen content of the vessels was determined by using a colorimetric hydroxyproline assay, as described in ref. 51.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 HL63766 and R21 HL081560. L.E.N. is a Beeson Physician Scholar and is supported by the American Federation for Aging Research. R.Y.K. is funded as a Howard Hughes Medical Student Research Fellow and American Heart Association Predoctoral Fellow.
Abbreviations
- hTERT
human telomerase reverse transcriptase
- PD
population doubling
- SKY
spectral karyotype
- SMC
smooth muscle cells
- yo
year-old.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Shieh S.-J., Vacanti J. Surgery. 2005;137:1–7. doi: 10.1016/j.surg.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Poh M., Boyer M., Solan A., Dahl S., Pedrotty D., Banik S., McKee J., Klinger R., Counter C., Niklason L. Lancet. 2005;365:2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 3.L'Heureux N., Paquet S., Labbe R., Germain L., Auger F. A. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Niklason L. E., Gao J., Abbott W. M., Hirschi K. K., Houser S., Marini R., Langer R. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 5.Thomas M., Lianqing Y., Hornsby P. J. Nat. Biotechnol. 2000;18:39–42. doi: 10.1038/71894. [DOI] [PubMed] [Google Scholar]
- 6.Seigneurin-Venin S., Bernard V., Tremblay J. P. Gene Ther. 2000;7:619–623. doi: 10.1038/sj.gt.3301132. [DOI] [PubMed] [Google Scholar]
- 7.Seigneurin-Venin S., Bernard V., Moisset P.-A., Ouellette M. M., Mouly V., Di Donna S., Wright W. E., Tremblay J. P. Biochem. Biophys. Res. Commun. 2000;272:362–369. doi: 10.1006/bbrc.2000.2735. [DOI] [PubMed] [Google Scholar]
- 8.Bodnar A. G., Ouellette M., Frolkis M., Holt S. E., Chiu C.-P., Morin G. B., Harley C. B., Shay J. W., Lichtsteiner S., Wright W. E. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 9.Counter C. M., Meyerson M., Eaton E. N., Ellison L. W., Caddle S. D., Haber D. A., Weinberg R. A. Oncogene. 1998;16:1217–1222. doi: 10.1038/sj.onc.1201882. [DOI] [PubMed] [Google Scholar]
- 10.Vaziri H., Benchimol S. Curr. Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 11.Jiang X.-R., Jimenez G., Chang E., Frolkis M., Kusler B., Sage M., Beeche M., Bodnar A. G., Wahl G. M., Tisty T. D., Chiu C.-P. Nat. Genet. 1999;21:111–114. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- 12.Morales C. P., Holt S. E., Ouellette M., Kaur K. J., Yan Y., Wilson K. S., White M. A., Wright W. E., Shay J. W. Nat. Genet. 1999;21:115–118. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- 13.Funk W. D., Wang C. K., Shelton D. N., Harley C. B., Pagon G. D., Hoeffler W. K. Exp. Cell Res. 2000;258:270–278. doi: 10.1006/excr.2000.4945. [DOI] [PubMed] [Google Scholar]
- 14.Wyllie F. S., Jones C. J., Skinner J. W., Haughton M. F., Wallis C., Wynford-Thomas D., Faragher R. G., Kipling D. Nat. Genet. 2000;24:16–17. doi: 10.1038/71630. [DOI] [PubMed] [Google Scholar]
- 15.Yang J., Chang E., Cherry A. M., Bangs C. D., Oei Y., Bodnar A., Bronstein A., Chiu C.-P., Herron G. S. J. Biol. Chem. 1999;274:26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- 16.Gu X., Zhang J., Brann D. W., Yu F. S. Invest. Ophthalmol. Visual Sci. 2003;44:3219–3225. doi: 10.1167/ivos.02-0852. [DOI] [PubMed] [Google Scholar]
- 17.Yang J., Nagavarapu U., Relloma K., Sjaastad M. D., Moss W. C., Passaniti A., Herron G. S. Nat. Biotechnol. 2001;19:219–224. doi: 10.1038/85655. [DOI] [PubMed] [Google Scholar]
- 18.Shi S., Gronthos S., Chen S., Reddi A., Counter C. M., Robey P. G., Wang C. Y. Nat. Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- 19.Simonsen J. L., Rosada C., Serakinci N., Justesen J., Stenderup K., Rattan S. I., Jensen T. G., Kassem M. Nat. Biotechnol. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 20.Gronthos S., Chen S., Wang C. Y., Robey P. G., Shi S. J. Bone Miner. Res. 2003;18:716–722. doi: 10.1359/jbmr.2003.18.4.716. [DOI] [PubMed] [Google Scholar]
- 21.Kawano Y., Kobune M., Yamaguchi M., Nakamura K., Ito Y., Sasaki K., Takahashi S., Nakamura T., Chiba H., Sato T., et al. Blood. 2003;101:532–540. doi: 10.1182/blood-2002-04-1268. [DOI] [PubMed] [Google Scholar]
- 22.Yudoh K., Matsuno H., Nakazawa F., Katayama R., Kimura T. J. Bone Miner. Res. 2001;16:1453–1464. doi: 10.1359/jbmr.2001.16.8.1453. [DOI] [PubMed] [Google Scholar]
- 23.Yudoh K., Nishioka K. Gene Ther. 2004;11:909–915. doi: 10.1038/sj.gt.3302234. [DOI] [PubMed] [Google Scholar]
- 24.Kobune M., Kawano Y., Ito Y., Chiba H., Nakamura K., Tsuda H., Sasaki K., Dehari H., Uchida H., Honmou O., et al. Exp. Hematol. (Charlottesville, VA) 2003;31:715–722. doi: 10.1016/s0301-472x(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 25.Schnabl B., Choi Y. H., Olsen J. C., Hagendorn C. H., Brenner D. A. Lab. Invest. 2002;82:323–333. doi: 10.1038/labinvest.3780426. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe T., Shibata N., Westerman K. A., Okitsu T., Allain J. E., Sakaguchi M., Totsugawa T., Maruyama M., Matsumura T., Noguchi H., et al. Transplantation. 2003;75:1873–1880. doi: 10.1097/01.TP.0000064621.50907.A6. [DOI] [PubMed] [Google Scholar]
- 27.Weinberg R. Sci. Am. 1996;275(3):62–70. doi: 10.1038/scientificamerican0996-62. [DOI] [PubMed] [Google Scholar]
- 28.Maser R., DePinho R. Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- 29.Mondello C., Chiesa M., Rebuzzini P., Zongaro S., Verri A., Colombo T., Giulotto E., D'Incalci M., Franceschi C., Nuzzo F. Biochem. Biophys. Res. Commun. 2003;308:914–921. doi: 10.1016/s0006-291x(03)01484-0. [DOI] [PubMed] [Google Scholar]
- 30.McKee J. A., Banik S. S., Boyer M. J., Hamad N. M., Lawson J. H., Niklason L. E., Counter C. M. EMBO Rep. 2003;4:633–638. doi: 10.1038/sj.embor.embor847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morales C. P., Gandia K. G., Ramirez R. D., Wright W. E., Shay J. W., Spechler S. J. Gut. 2003;52:327–333. doi: 10.1136/gut.52.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luiten R. M., Pene J., Yssel H., Spits H. Blood. 2003;101:4512–4519. doi: 10.1182/blood-2002-07-2018. [DOI] [PubMed] [Google Scholar]
- 33.O'Hare M. J., Bond J., Clarke C., Takeuchi Y., Atherton A. J., Berry C., Moody J., Silver A. R. J., Davies D. C., Alsop A. E., et al. Proc. Natl. Acad. Sci. USA. 2001;98:646–651. doi: 10.1073/pnas.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn W. C., Counter C. M., Lundberg A. S., Beijersbergen R. L., Brooks M. W., Weinberg R. A. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 35.Harley C. Oncogene. 2002;21:494–502. doi: 10.1038/sj.onc.1205076. [DOI] [PubMed] [Google Scholar]
- 36.Wooton M., Steeghs K., Watt D., Munro J., Gordon K., Ireland H., Morrison V., Behan W., Parkinson E. Hum. Gene Ther. 2003;14:1473–1487. doi: 10.1089/104303403769211682. [DOI] [PubMed] [Google Scholar]
- 37.Milyavsky M., Shats I., Erez N., Tang X., Senderovich S., Meerson A., Tabach Y., Goldfinger N., Ginsberg D., Harris C., Rotter V. Cancer Res. 2003;63:7147–7157. [PubMed] [Google Scholar]
- 38.Burns J., Abdallah B., Guldberg P., Rygaard J., Schroder H., Kassem M. Cancer Res. 2005;65:3126–3135. doi: 10.1158/0008-5472.CAN-04-2218. [DOI] [PubMed] [Google Scholar]
- 39.Serakinci N., Guldberg P., Burns J., Adballah B., Schrodder H., Jensen T., Kassem M. Oncogene. 2004;23:5095–5098. doi: 10.1038/sj.onc.1207651. [DOI] [PubMed] [Google Scholar]
- 40.Leach N., Rehder C., Jensen K., Holt S., Jackson-Cook C. Mech. Ageing Dev. 2004;125:563–573. doi: 10.1016/j.mad.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Reix T., Sevestre H., Sevestri-Pietri M.-A., Szychta P., Pietri J. Ann. Vasc. Surg. 1998;12:589–596. doi: 10.1007/s100169900205. [DOI] [PubMed] [Google Scholar]
- 42.Blansfield J., Chung H., Sullivan T., Pezzi C. Ann. Vasc. Surg. 2003;17:565–570. doi: 10.1007/s10016-003-0038-6. [DOI] [PubMed] [Google Scholar]
- 43.Kim S., Kaminker P., Campisi J. Oncogene. 2002;21:503–511. doi: 10.1038/sj.onc.1205077. [DOI] [PubMed] [Google Scholar]
- 44.Armbruster B., Linardic C., Veldman T., Bansal N., Downie D., Counter C. Mol. Cell. Biol. 2004;24:3552–3561. doi: 10.1128/MCB.24.8.3552-3561.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niklason L. E., Abbott W., Gao J., Klagges B., Hirschi K. K., Ulubayram K., Conroy N., Jones R., Vasanawala A., Sanzgiri S., Langer R. J. Vasc. Surg. 2001;33:628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 46.Matsui S., Faitar S., Rossi M., Cowell J. Cancer Genet. Cytogenet. 2003;142:30–35. doi: 10.1016/s0165-4608(02)00730-6. [DOI] [PubMed] [Google Scholar]
- 47.Matsui S., Sait S., Jones C., Nowak N., Gross K. Mamm. Genome. 2002;13:680–685. doi: 10.1007/s00335-002-2197-0. [DOI] [PubMed] [Google Scholar]
- 48.Ried T., Koehler M., Padilla-Nash H., Shroek E. In: Cells: A Laboratory Manual. Spector D., Goldman R., Leinwand L., editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. [Google Scholar]
- 49.Ried T., Liyanage M., du Manoir S., Heselmeyer K., Auer G., Macville M., Shroek E. J. Mol. Med. 1997;75:801–814. doi: 10.1007/s001090050169. [DOI] [PubMed] [Google Scholar]
- 50.Cifone M., Fidler I. Proc. Natl. Acad. Sci. USA. 1980;77:1039–1043. doi: 10.1073/pnas.77.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahl S. L., Koh J., Prabhakar V., Niklason L. E. Cell Transplant. 2003;12:659–666. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.