Abstract
The present research was undertaken to determine the conformations of alkyl groups that are forced into small spaces. Unbranched alkyl groups assume fully extended conformations in the free space of solutions, because any bends in the structure create steric clashes between hydrogens along the chain. We synthesized a series of alkyl esters of a carboxylic acid attached to the inner surface of a vase-shaped container structure. The shorter esters (ethyl and propyl) can be accommodated in extended conformations, and even small solvent molecules can share the container’s space. Longer (butyl-octyl) esters adopt increasingly coiled conformations that writhe rapidly in the limited space of the cavity. Even longer esters (nonyl and decyl) can be synthesized, but their containers become distorted, and their spectra indicate slowed internal motions of the alkyl groups within the space. In general, alkyl groups are readily contorted when their internal strains are compensated by attraction with the inner surfaces and the proper filling of space.
Keywords: coiled conformations, self-assembly
The lowest energy conformation of normal alkanes is a fully extended (anti) one in solution, the gas phase, and the solid state. Each bend creates gauche interactions involving repulsion between hydrogens on the Ci and Ci+3 carbons, and each gauche configuration is associated with an increase in energy of ≈0.55 kcal/mol in the liquid state (1). The small energy involved means that, for a given alkane, a number of conformers are typically present in equilibrium. For example, n-heptane has 13 different accessible conformers in rapid interconversion under ambient conditions (2).
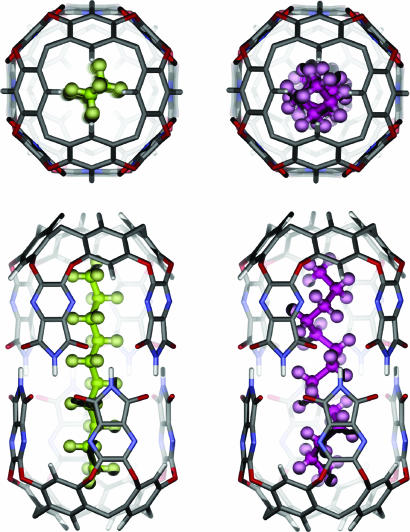
The recent literature describes several systems in which the energetic preference for the anti conformation may be overcome by imposing geometric constraints on the size and shape of the space available (3–11). Naturally occurring protein receptors (12) and synthetic host molecules contain binding cavities suitable for hydrocarbons, and a variety of conformations have been observed by crystallography in the former and NMR in the latter. Normal alkanes are simply flexible and adept at filling spaces of diverse shape. We have examined normal alkanes in several hosts: hexameric pyrogallolarene assemblies encompassing a roughly cube-like space of ≈1,300 Å3 (11); vase-shaped, water-soluble cavitands having an open-ended pocket of ≈200 Å3 (7, 8); and a cylindrical molecular capsule having an inner space of 425 Å3 (6, 9). The behavior in these systems fits neither Fischer’s (13) lock-and-key model nor the Thoma and Koshland (14) induced-fit model: the alkane contorts to be accommodated; it assumes the size, shape, and chemical surface that are proper to the space on offer in the receptor. For example, n-alkanes shorter than the cavity of the cylinder (Fig. 1) are bound in an extended conformation; if the alkanes are slightly too long, they adopt a compact helical conformation with several gauche configurations. This conformation is shorter and thicker: it allows the alkane to fit the cavity’s length and width; CH–π interactions between the guest and the aromatic surfaces of the host are also facilitated. The n-alkanes that contract this way are often those bound with the highest affinities. If the alkanes are too long (even in their coiled conformations), they are simply not encapsulated.
Fig. 1.
Alkanes adopt a conformation suitable for the available space. (Left) Two views of encapsulated decane in an extended conformation. (Right) Encapsulated tetradecane coils into a helical conformation.
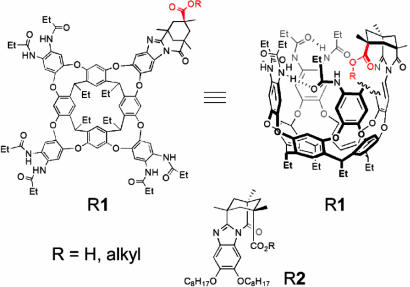
Here, we examine the behavior of a series of alkanes that are covalently attached to a receptor as ester groups (Fig. 2). Because the carboxyl of the ester is introverted, by which we mean directed into the cavity, the alkyl groups have no choice; they are forced to be accommodated by whatever conformations are available. Many of these examples involve an alkyl group that, in its extended anti conformation, exceeds the length of the cavity’s dimensions, and we report here the consequences.
Fig. 2.
Structure of the introverted acid cavitand H1, the introverted esters R1, and control molecules 2.
Results and Discussion
The introverted ester cavitands are prepared from the introverted acid cavitand, which has been reported (Fig. 2) (15–17). The introverted acid cavitand is a vase-shaped host, with cavity dimensions of ≈6 Å between the van der Waals surfaces of opposite walls and between the deepest part of the cavity and the amide carbonyls of the upper rim. The idealized conformation shown in the line drawing has a volume of ≈170 Å3 (18); a guest molecule typically occupies around half of this space. The nine-membered diether rings impart flexibility on the cavitand, allowing the walls to draw closer or move farther from the center of the cavity in response to a guest (19). The amide groups of the upper rim stabilize this vase conformation by forming a seam of hydrogen bonds in either a clockwise or counterclockwise orientation (20, 21) determined by the handedness of the N-acylbenzimidazole. The plasticity of hydrogen bonds permits extensive “breathing” at the upper rim. Although the cavitand is chiral, it is not particularly asymmetric in a steric sense because the acylbenzimidazole and the amides that are arranged by it present their flat surfaces to the cavity and the guests within. The cavity does have magnetic asymmetry because the flat parts present loosely held electrons in π bonds. The cavitand is synthesized as a racemic mixture.
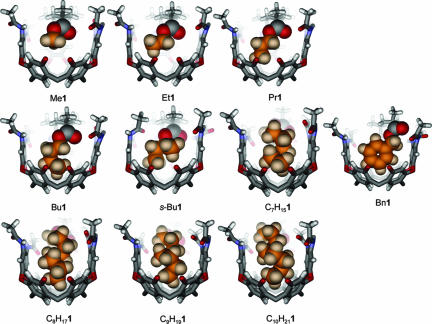
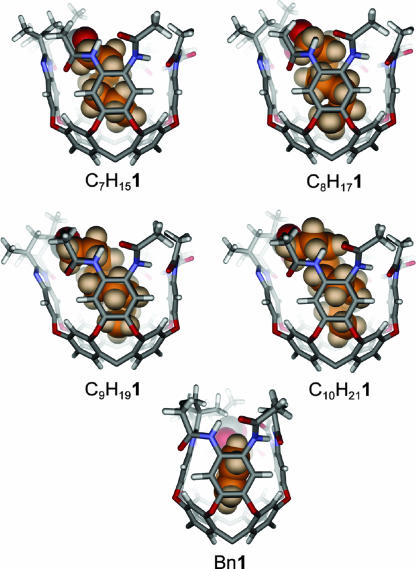
Molecular modeling (maestro; Schrödinger, Portland, OR; AMBER force field) provided energy-minimized structures of the cavitands (Fig. 3). The minimizations were carried out starting from coiled ester chains, which are consistent with the NMR studies described below. Only the shortest esters, up to propyl, are accommodated in a fully extended conformation by the cavitand without severe steric clashes. The model of the introverted butyl ester Bu1 minimized with a gauche configuration in its ester chain from a starting structure that was fully anti. This step is the beginning of coiling. The heptyl ester C7H151 and longer introverted esters have tightly coiled conformations that arise from the conversion of most or all of their C–C–C–C dihedral angles from anti to gauche. The diameter of a helical alkane is ≈5.5 Å, and such a coil can make good van der Waals contact with the walls of the cavitand, stabilized by CH–π interactions. With a distance of only 5 Å between the ester oxygen atoms and the bottom of the cavitand, the coiled conformation places each methylene unit of the alkyl chain progressively deeper in the cavity.
Fig. 3.
Molecular models (maestro; AMBER force field) of the introverted ester cavitands R1.
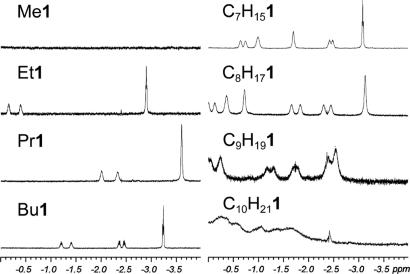
The NMR spectra of the introverted ester cavitands in mesitylene-d12 are consistent with the model structures (Figs. 3 and 4). The methyl and methylene hydrogens of the ester chains lie in the shielding region of the anisotropic magnetic environment of the cavitand arenes, and most of them appear upfield of 0 ppm where they are clearly resolved. In all of the spectra, the terminal methyl group has the farthest upfield resonance, and it appears that a folded, hairpin-like chain conformation cannot be accommodated. None has a farther upfield chemical shift than that of the propyl ester Pr1, which is likely in a fully extended conformation. The chirality of the cavitand is manifest in the diastereotopic differentiation of the geminal hydrogens of the ester chain. Internal motions, such as rotation of the carboxyl group and interconversion of various coiled conformations, are relatively rapid on the NMR timescale.
Fig. 4.
The upfield window of the 1H NMR spectra of linear alkyl introverted ester cavitands. The solvent is mesitylene-d12. The spectra of Me1, Et1, Pr1, and Bu1 were acquired at 300 K; the others were acquired at 273 K.
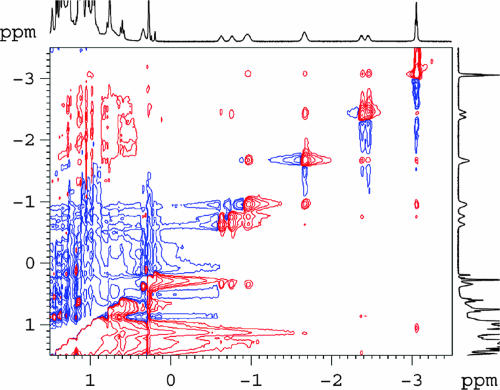
The NMR spectra confirm that each methylene group from ester oxygen to the terminal methyl group is progressively deeper in the cavitand. In a COSY spectrum of heptyl ester C7H151 (see Fig. 8, which is published as supporting information on the PNAS web site), the 1H resonance of each ester methylene group has 3J coupling to only the closest resonance upfield and the closest resonance downfield. By using this spectrum, the 1H signals of the ester can be fully assigned. With its relatively sharp signals, the heptyl ester C7H151 gave a clear NOESY spectrum (Fig. 5). The terminal ester methyl group has a stronger nuclear Overhauser effect (NOE) to Ci+3 than to Ci+2, in addition to a small NOE to Ci+4. A similar pattern is seen for the methylene group next to the terminal methyl group. These NOEs are indicative of the coiled conformation of the ester chain.
Fig. 5.
NOESY spectrum of heptyl ester C7H151 at 273 K in mesitylene-d12. A NOE between the terminal methyl group at −3.1 ppm and (CH2)i+4 at −0.7 ppm is indicative of coiling.
The downfield region of the 1H spectra of the esters differs significantly (see Fig. 9, which is published as supporting information on the PNAS web site). This region of the spectra contains the amide NH signals, which are responsive to the conformation of the cavitand. Although no clear pattern of change is evident, there are significant structural differences between introverted ester cavitands that differ in ester length by only a methylene group. The molecular models (Figs. 3 and 6) provide a reasonable explanation. Because of the limited dimensions of the cavity, the largest introverted esters deform the cavitand in a way that provides more space. The N-acylbenzimidazole wall of the cavitand is tilted progressively outward with increasing length of the ester chain, until a limit is reached at the nonyl ester C9H181. Its proton spectrum, acquired at 273 K, has resolved ester signals. In contrast, we were unable to obtain a sharp spectrum of the decyl ester C10H211 at any temperature between 240 and 323 K. The cavitand is unfolded; the chemical shift of the cavitand methine resonance confirms this finding (19).
Fig. 6.
Side views of the molecular models (maestro; AMBER force field) of selected introverted ester cavitands. The decyl ester C10H211 is modeled in its folded conformation for illustrative purposes; NMR structural studies show that the ester chain is too large, and the cavitand is unfolded.
There is a mutual adaptation of cavitand and ester chain in response to the limited size of the inner space. We used the grasp program (http://trantor.bioc.columbia.edu/grasp) to calculate the inner van der Waals volume of the cavitand with the ester chain removed and to calculate the volume of the excised ester. The ratio of these volumes, the packing coefficient, quantifies the efficiency of space filling (Table 1). The shortest esters have a low packing coefficient but probably also contain atmospheric gases; however, as the chain length is increased, the packing coefficient converges on 0.60 with a standard deviation of 0.03. As the cavitand walls tilt inward or outward in response to ester size, the volume of the cavitand’s inner space changes from ≈115 to ≈250 Å3, all while maintaining this packing coefficient. The addition of a methylene group increases the van der Waals volume of the ester chain by 16 Å3 and is accommodated by an increase in cavitand volume by an average of 26 Å3. The ester chain maintains its coiled conformation with its terminal methyl group deepest in the cavitand throughout the series until the decyl ester is reached: There the cavitand can accommodate no more and “blows up.” At this point, the increasing steric demands of the ester chain have overcome the network of amide hydrogen bonds that stabilizes the folded conformation of the cavitand.
Table 1.
Packing coefficients of introverted esters
| Ester | Cavity volume, Å3 | Included group* volume, Å3 | PC |
|---|---|---|---|
| Me1.20 | 93 | 23 | 0.25 |
| Et1.20 | 91 | 37 | 0.41 |
| Pr1.20 | 100 | 53 | 0.52 |
| Bu1.20 | 110 | 69 | 0.64 |
| sBu1.20 | 120 | 68 | 0.59 |
| C7H151.20 | 200 | 120 | 0.57 |
| C8H171.20 | 220 | 130 | 0.62 |
| C9H191.20 | 250 | 150 | 0.59 |
| Bn1.20 | 140 | 92 | 0.67 |
| CHCl3·Et1.20 | 130 | 68† | 0.52 |
| CHCl3·Pr1.20 | 130 | 68† | 0.52 |
Volumes of ester chains and cavities (minimized with maestro using the AMBER force field) were determined using the grasp program (20).
*The included group is the ester chain, except when CHCl3 is present (†).
The maximum packing coefficient of an ester chain in this cavitand is ≈0.60; we propose that this value represents the upper limit of the ability of an n-alkane to compact itself through coiling in response to a limited space. For comparison, we refer to the study of the encapsulation of n-alkanes in the cylindrical molecular capsule (Fig. 1) (9). The longest alkane bound, tetradecane, adopted a coiled conformation, stretched the hydrogen-bonded seam between the capsule halves, and approached this limit of space filling with a packing coefficient of 0.57. Pentadecane, with a theoretical packing coefficient of 0.61, was too large and did not bind. Coiling may be the most effective means of increasing hydrocarbon density. Docosane, a wax at room temperature, has a packing coefficient of 0.55 (the packing coefficient of docosane is calculated by taking the van der Waals volume of one molecule, multiplying it by the number of molecules in 1 liter, and dividing it by 1 liter), 9% less than that achieved in these cavitands.
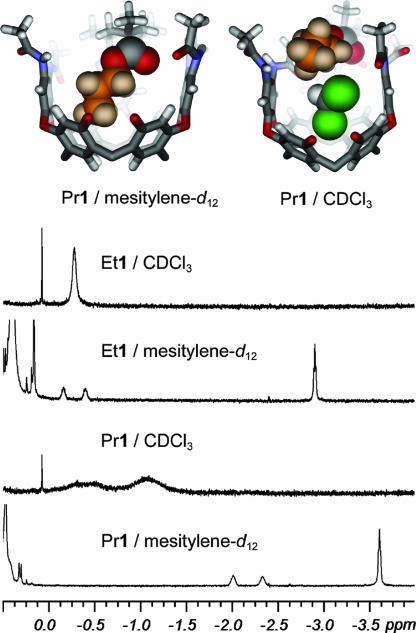
The introverted orientation of the acid in H1 and the esters in R1 has consequences for their behavior on thin-layer chromatography (TLC). For example, the acid H1 and the methyl ester Me1 cannot be distinguished by TLC (1:9 CH2Cl2:EtOAc), but longer esters such as C7H151 are retained less by the silica and are easily resolved. The small functional groups, acid and methyl ester, are shielded from interactions with silica by the walls of the cavitand; the longer esters may contort the cavitand (as in Fig. 6), and they may also be displaced from the cavity of 1 by some solvent molecules (as described below; see Fig. 7). The contortion or the displacement (or both) would account for the difference in chromatographic retention. This result is similar to one reported recently by Liu and Warmuth (22), in which a hemicarcerand changes its volume and shape in response to different guest sizes, and the interactions with a chromatographic stationary phase change accordingly.
Fig. 7.
1H NMR spectra (Lower) and model structures (Upper) of introverted ester cavitands in mesitylene-d12 and CDCl3. The upfield region of the NMR spectra, shown in Left Upper, indicate that the ester chain is displaced from the cavity by solvents of appropriate size and shape, in this case chloroform, as shown in Right Upper.
The diastereomeric introverted sec-butyl esters s-Bu1 (Fig. 3) were surprisingly differentiated by chromatography despite the close similarity of their structures (at least when viewed from outside the cavitand). They were indistinguishable by TLC, but HPLC (βsil C18 column; 4.6 × 250 mm; 100% CH3OH; 0.5 ml/min) had sufficient resolution to separate them. We propose that these diastereomers affect the shapes of the cavitands slightly: enough to allow their separation. The NMR spectrum of the mixture of diastereomers, which were synthesized by treating H1 with diazo-sec-butane, showed that the diastereomeric excess (d.e.) was 20% (see Fig. 10, which is published as supporting information on the PNAS web site). A similar preparation using a control acid H2 resulted in a product mixture with a d.e. of 5%. The cavitand is only slightly more selective, but it does improve the differentiation between the methyl and ethyl groups of diazo-sec-butane in the transition state.
The introverted benzyl ester Bn1 (Figs. 3 and 6) lacks the conformational variability intrinsic to the other esters; most of the conformational adaptation in this case comes from the cavitand. The modeling studies suggest that the walls of the cavitand tilt in such a fashion that the N-acylbenzimidazole wall and the wall opposite make face-to-face π–π contacts with the benzyl group, and the remaining cavitand walls make edge-to-face contacts with the same. The benzyl ester cavitand exemplifies the way in which a functional group may be spatially isolated from a reaction. We treated a sample of Bn1 with H2 in the presence of Pd/C in mesitylene and compared its hydrogenolysis with that of Bn2. The control molecule Bn2 was smoothly converted to the acid H2 in <20 min, whereas, using TLC, we estimated 5% conversion of Bn1 to H1 after 24 h. The introverted nature of the esters of 1 has repercussions in terms of conformation and reactivity.
Conclusions
We have synthesized and studied a series of introverted ester cavitands of increasing ester chain length. The propyl ester Pr1 is the shortest that can have an anti staggered conformation; the longer esters coil in response to the limited volume of the inner space. The coiling of alkanes is a natural result of the conversion of anti configurations to gauche: the distance between methylene groups is shortened, and NOE measurements can be used for characterization. By studying the deformation of the cavitand in response to increasing ester chain length, we have shown that the packing coefficient reaches a maximum of ≈0.60. Esters longer than nine carbons do not compact more densely; they disrupt the folded conformation of the cavitand. We propose that coiling maximizes the density of a hydrocarbon and may be the most efficient response to limited space. Small solvent molecules such as chloroform are able to displace the ester chain from the cavitand: a host–guest complex results.
The packing of the ester chain in the inner space of the cavitand leads to deformations of the cavitand’s upper rim, which can be sensed chromotographically. The methyl ester Me1 and the acid H1 are indistinguishable by TLC, whereas the heptyl ester and those longer are less retained by the silica. HPLC had sufficient resolution to separate the epimers of the sec-butyl ester s-Bu1; we attribute this result to a small difference in the shape of the cavitand. The benzyl ester Bn1 deforms the cavitand so as to allow both edge-to-face and face-to-face π–π interactions. The sheltered environment of this benzyl ester attenuates its reactivity under conditions of Pd-catalyzed hydrogenolysis.
Methods
The introvert ester cavitands are synthesized by the esterification of the introverted acid cavitand H1 (Fig. 2) with the respective diazoalkanes. Diazomethane was prepared as an ethereal solution by using the Diazald precursor and an Aldrich Mini Diazald Apparatus, as described in that product’s literature. Other linear diazoalkanes (Et, Pr, Bu, C7H15, C8H17, C9H19, C10H21, and Bn) were prepared from the precursors and according to the methods described by Sekiya and coworkers (23). Of these, the Et, Pr, and Bu diazoalkanes were codistilled with ether; the others were prepared as a solution in cyclohexane. Diazo-sec-butane cannot be prepared from the N-[(N-nitrosoalkylamino)methyl]carbamate precursors of Sekiya but is derived by the HgO oxidation of butan-2-ylidenehydrazine and is codistilled as an ethereal solution (24).
In a typical preparation, a sample of 10 mg of H1 is dissolved in 2 ml of CH2Cl2. The freshly prepared solution of diazoalkane is added in excess (so that the color persists), and the reaction is mixed by a brief swirling of the flask. After 3 min, analysis by TLC [CH2Cl2:EtOAc (1:9)] or NMR indicated that the reaction was complete in all cases. The C7H15, C8H17, C9H19, C10H21, and Bn esters were purified by preparative TLC [CH2Cl2:EtOAc (1:9)]. The others were pure after evaporation of the esterification reaction mixture; the diazoalkanes from which they are derived are available in high purity because they are distilled. The esterifications were high yielding, although some material was then lost in preparative TLC. The NMR spectra are shown in full in Figs. 11–24, which are published as supporting information on the PNAS web site. High-resolution mass spectra confirm the identity of the compounds.
Supplementary Material
Acknowledgments
This work was supported by The Skaggs Institute for Chemical Biology and National Institutes of Health Grant GM27932. B.W.P. is a Skaggs Predoctoral Fellow.
Abbreviation
- NOE
nuclear Overhauser effect.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Eliel E. L., Wilen S. H. Stereochemistry of Organic Compounds. New York: Wiley; 1994. [Google Scholar]
- 2.Dale J. Stereochemistry and Conformational Analysis. New York: Verlag Chemie; 1978. [Google Scholar]
- 3.Seneque O., Rager M.-N., Giorgi M., Reinaud O. J. Am. Chem. Soc. 2000;122:6183–6189. doi: 10.1021/ja016345e. [DOI] [PubMed] [Google Scholar]
- 4.Udachin K. A., Enright G. D., Brouwer E. B., Ripmeester J. A. J. Supramol. Chem. 2001;1:97–100. [Google Scholar]
- 5.Stahl J., Bohling J. C., Bauer E. B., Peters T. B., Mohr W., Martin-Alvarez J. M., Hampel F., Gladysz J. A. Angew. Chem. Int. Ed. 2002;41:1871–1876. [PubMed] [Google Scholar]
- 6.Hayashida O., Sebo L., Rebek J., Jr J. Org. Chem. 2002;67:8291–8298. doi: 10.1021/jo0201171. [DOI] [PubMed] [Google Scholar]
- 7.Trembleau L., Rebek J., Jr Science. 2003;301:1219–1221. doi: 10.1126/science.1086644. [DOI] [PubMed] [Google Scholar]
- 8.Trembleau L., Rebek J., Jr Chem. Commun. 2004:58–59. doi: 10.1039/b311424d. [DOI] [PubMed] [Google Scholar]
- 9.Scarso A., Trembleau L., Rebek J., Jr Angew. Chem. Int. Ed. 2003;42:5499–5502. doi: 10.1002/anie.200352235. [DOI] [PubMed] [Google Scholar]
- 10.Purse B. W., Rebek J., Jr Chem. Commun. 2005:722–724. doi: 10.1039/b408878f. [DOI] [PubMed] [Google Scholar]
- 11.Palmer L. C., Rebek J., Jr Org. Lett. 2005;7:787–789. doi: 10.1021/ol047673x. [DOI] [PubMed] [Google Scholar]
- 12.Han G. W., Lee J. Y., Song H. K., Chang C., Min K., Moon J., Shin D. H., Kopka M. L., Sawaya M. R., Yuan H. S., et al. J. Mol. Biol. 2001;308:263–278. doi: 10.1006/jmbi.2001.4559. [DOI] [PubMed] [Google Scholar]
- 13.Fischer E. Berichte der Deutschen Chemischen Gesellschaft. 1894;27:2985. [Google Scholar]
- 14.Thoma J. A., Koshland D. E., Jr J. Am. Chem. Soc. 1960;82:3329–3333. [Google Scholar]
- 15.Renslo A. R., Rebek J., Jr Angew. Chem. Int. Ed. 2000;39:3281–3283. doi: 10.1002/1521-3773(20000915)39:18<3281::aid-anie3281>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Wash P. L., Renslo A. R., Rebek J., Jr Angew. Chem. Int. Ed. 2001;40:1221–1222. [PubMed] [Google Scholar]
- 17.Purse B. W., Ballester P., Rebek J., Jr J. Am. Chem. Soc. 2003;125:14682–14683. doi: 10.1021/ja036595q. [DOI] [PubMed] [Google Scholar]
- 18.Nicholls A., Sharp K. A., Honig B. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 19.Moran J., Ericson J., Dalcanale E., Bryant J., Knobler C., Cram D. J. Am. Chem. Soc. 1991;113:5707–5714. [Google Scholar]
- 20.Rudkevich D. M., Hilmersson G., Rebek J., Jr J. Am. Chem. Soc. 1997;119:9911–9912. [Google Scholar]
- 21.Rudkevich D. M., Hilmersson G., Rebek J., Jr J. Am. Chem. Soc. 1998;120:12216–12225. [Google Scholar]
- 22.Liu Y., Warmuth R. Angew. Chem. Int. Ed. 2005;44:7107–7110. doi: 10.1002/anie.200501840. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita H., Ito K., Terao Y., Sekiya M. Chem. Pharm. Bull. 1979;27:682–687. [Google Scholar]
- 24.Andrews S. D., Day A. C., Raymond P., Whiting M. C. Org. Syntheses. 1988;6:392–394. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.