Abstract
For many pathogens, cell adhesion factors are critical virulence determinants. Enteropathogenic Yersinia species express the afimbrial adhesin YadA, the prototype of a class of homotrimeric outer membrane adhesins, which mediates adherence to host cells by binding to extracellular matrix components. In this study, we demonstrate that different pathogenic functions are attributable to highly homologous YadA proteins. YadA of Yersinia pseudotuberculosis (YadApstb) and Yersinia enterocolitica (YadAent) exhibit fundamental differences in their specificity of extracellular matrix substrate binding, they cause dissimilar bacterial aggregation behaviors, and YadApstb, but not YadAent, promotes efficient uptake into human cells. Evidence is presented here that a unique N-terminal amino acid sequence of YadApstb, which is absent in YadAent, acts as an “uptake domain” by mediating tight binding to fibronectin bound on α5β1 integrin receptors, which are crucial for initiating the entry process. Deleting this motif in YadApstb generated all features of the YadAent protein, i.e., the molecule lost its adhesiveness to fibronectin and its invasiveness, but gained adhesion potential to collagen and laminin. Loss of the “uptake region” also attenuated host tissue colonization by Y. pseudotuberculosis during oral infections of mice, demonstrating that this motif plays a crucial role in defining pathogen–host cell interaction and pathogenesis. We conclude that even small variations in adhesion factors can provoke major differences in the virulence properties of related pathogens.
Keywords: bacterial pathogenesis, host–pathogen interaction, multifunctional adhesin
The enteropathogenic bacteria Yersinia enterocolitica and Yersinia pseudotuberculosis cause a number of enteric diseases ranging from enteritis and lymphadenitis to autoimmune disorders (1). They express nonfimbrial surface proteins that promote cell attachment and translocation across the intestinal layer via M cells and permit colonization of underlying lymphatic tissues and organs (2, 3). The most efficient strategy of Yersinia to bind and penetrate M cells involves the invasin protein, which promotes cell entry by binding to different β1 integrin receptors found on the surface of most mammalian cells (4, 5). An alternative internalization pathway seems to involve YadA, a virulence plasmid (pIB1)-encoded outer membrane adhesin (6–8).
The structure of the YadA protein of Y. enterocolitica (YadAent) has been investigated in detail (9). YadAent proteins form a capsule-like structure of trimeric “lollipop”-shaped surface projections that densely cover the bacterial surface. They consist of a bulky N-terminal head domain, which creates an intrinsically stable trimeric “lock-nut” structure, a coiled-coil pillar-like intermediate stalk, and a C-terminal membrane anchor domain (10–12). Recent identification of homologous outer membrane proteins in other pathogens, including Bartonella, Xanthomonas, Moxarella and Neisseria spp., suggested that YadA is the prototype of a class of afimbrial adhesins with important virulence traits essential for establishing infections (13–15).
The YadA protein is multifunctional and promotes binding to epithelial cells, professional phagocytes, and various extracellular matrix (ECM) molecules, such as laminin, collagen, and fibronectin. Expression of YadA also causes bacterial aggregation and induces specific agglutination of erythrocytes (16–19). Furthermore, YadA protects Yersinia against defensins and confers resistance to the bactericidal activity of serum complement (16). Structural analysis of YadAent further revealed that different portions of the molecule seem to be responsible for the specific biological properties of the adhesin. Serum resistance seems to be mediated by the stalk domain (11). The binding module(s) promoting adherence to neutrophils seem to be located in the extreme N-terminal part of the head region (16), whereas the collagen-binding activity resides within the central and C-terminal portion of the head domain of YadAent (20–22). Information about other binding functions is still missing. Several studies also demonstrated that YadA of Y. pseudotuberculosis (YadApstb) promotes efficient internalization of bacteria into human cells, and this process requires host cell β1 integrins, similar to the invasin protein (6–8). However, no uptake was reported with bacteria expressing Y. enterocolitica YadA (23, 24). The reason for this observation was unclear. In this study, we directly compared the structure and different functions of the two homologous adhesin molecules and identified an internal region in the head domain of YadApstb, which is absent in YadAent, that is critical for YadA-mediated cell aggregation, ECM specific substrate recognition, and bacterial internalization into human cells.
Results
Identification of an “Uptake Region” Within YadApstb.
To compare YadA-dependent entry of Y. enterocolitica and Y. pseudotuberculosis directly, we cloned yadApstb and yadAent under the control of the arabinose-inducible promoter PBAD and expressed the proteins in Escherichia coli K-12 and Y. pseudotuberculosis strain YP31 (pIB1−, inv−). This approach allowed yadA transcription in the absence of the virulence plasmid-encoded activator protein VirF and permitted the analysis of YadA function without other interfering components (e.g., invasin, Yops) known to affect uptake. Indirect immunofluorescence showed that both YadA proteins were surface-exposed, and the analysis of bacterial cell envelope preparations demonstrated that comparable amounts of YadApstb and YadAent were incorporated into the outer membranes of E. coli (data not shown) and Yersinia (Fig. 1A). Similar to previous results (6, 11), both proteins formed stable multimers of 180–200 kDa that could not be resolved by addition of sample buffer. To quantitatively evaluate their ability to promote bacterial attachment and entry into human cells, we used the bacteria to infect HEp-2 monolayers. The ability of cell binding was conferred on E. coli K-12 and YP31 by both YadA proteins (Fig. 1B). This result is in agreement with previous studies, demonstrating a strong interaction for both YadApstb and YadAent to various human cell types (7, 8, 23, 25). A considerable number (3–5%) of the YadApstb-expressing bacteria were internalized into cells 1 h after infection (Fig. 1C). In contrast, no significant levels of cell invasion (P < 0.0001) could be detected with the YadAent protein, even when time of infection was elongated. This result indicated that the YadA protein of Y. enterocolitica represents a highly efficient adhesin missing a feature of the YadApstb molecule required for uptake.
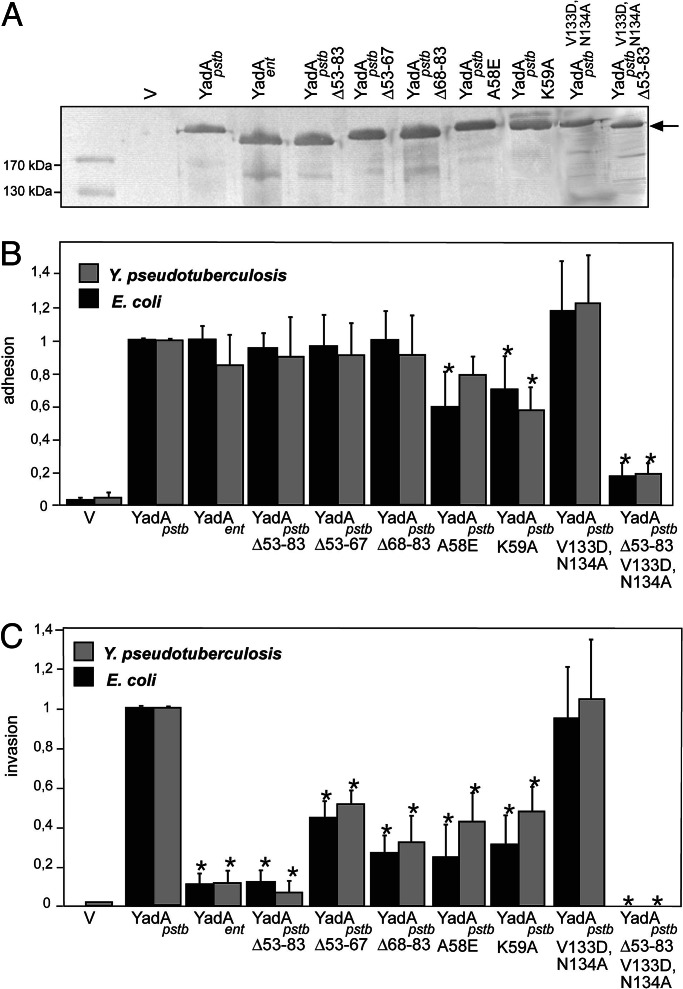
Fig. 1.
Outer membrane incorporation, adhesion, and invasion properties of the YadA proteins. (A) Immunoblot analysis demonstrates that comparable amounts of multimeric YadA derivatives (180–200 kDa) are expressed in the outer membrane of Yersinia strain YP31. V, empty vector. Cell adhesion (B) and cell invasion (C) of YadA-expressing E. coli and Y. pseudotuberculosis YP31 recombinant strains are documented. Approximately 106 bacteria were used to challenge 5 × 104 HEp-2 cells. Total numbers of adherent and intracellular bacteria are expressed relative to the adhesion and invasion rate of E. coli and Y. pseudotuberculosis YP31 harboring yadApstb, defined as 1.0. Each value represents the mean of at least three independent assays done in triplicate. Data were analyzed by the Student t test. ∗, significantly different from YadApstb-expressing bacteria (P < 0.05).
Sequence comparison revealed two major differences between the YadApstb and YadAent proteins: (i) deletions of several copies of a repeating region in the stalk domain of YadApstb, reducing the size of YadA extruding from the outer membrane (Fig. 5, which is published as supporting information on the PNAS web site), and (ii) 31 additional amino acids in the head domain of YadApstb that are absent in YadA of all known Y. enterocolitica serotypes (Fig. 2). Because the head domain has been shown to be involved in neutrophil interaction and ECM binding (22), we first investigated whether the unique 31-aa region in YadApstb is required for cell entry. To do so, a deletion derivative (yadApstbΔ53–83) resembling yadAent was constructed and expressed in E. coli K-12 (data not shown) and Yersinia YP31 (Fig. 1A). A considerable amount of YadApstbΔ53–83 was visualized in both strains and showed the expected size difference compared with the YadApstb protein. Obviously, the deletion had no negative effect on polymer formation or surface expression. Adhesion and invasion experiments of YadApstbΔ53–83 expressing E. coli or Yersinia further demonstrated that cell binding by YadApstbΔ53–83 was identical to YadAent and YadApstb, whereas cell entry was significantly impaired (P < 0.0001), similar to YadAent (Fig. 1 B and C). Given that a deletion of 31 aa in the YadApstb head region dissects bacterial adhesion from cell invasion indicates that this particular sequence of YadApstb acts as an “uptake domain.”
Fig. 2.
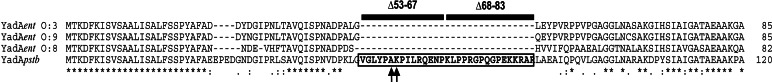
clustalw alignment of the head domain of YadA of Y. enterocolitica Serotype O:3 (YadAent O:3), O:9 (YadAent O:9) and O:8 (YadAent O:8), and Y. pseudotuberculosis Type III (YadApstb). Asterisks indicate amino acid identity, and dots indicate amino acid similarity. The additional sequence in YadA of Y. pseudotuberculosis, deleted in the YadApstbΔ53–83 protein, is indicated with two adjacent black boxes, which also illustrate the portions of the smaller deletions in YadApstbΔ53–67 and YadApstbΔ68–83. Exchanged amino acids A58E, K59A in the head domain of the YadApstb mutant derivatives are indicated by arrows.
Amino Acids Within the YadA Uptake Domain Are Crucial for Cell Invasion.
To identify elements within the 53–83 aa region that are critical for YadApstb-mediated cell entry, we generated a collection of point mutations within the putative uptake domain. Primarily charged amino acids and proline residues were chosen for mutagenesis, because they are important in other bacterial ligand–receptor interactions (26, 27). However, most point mutations had no effect on YadApstb-mediated cell entry. Only two adjacent substitutions (A58E and K59A) in the 53- to 83-aa region were found to reduce YadApstb-mediated cell penetration, but in their case, cell adhesion was also lowered to ≈60–70% (Fig. 1B). We also analyzed whether shorter deletions of codons 53–67 (Δ53–67) and codons 68–83 (Δ68–83) eliminate the uptake function. Both YadApstb deletion variants were expressed and promoted HEp-2 cell binding at levels similar to the YadApstb WT protein, but their capacity to mediate cell uptake was clearly reduced to ≈30–50% (Fig. 1C). This finding suggests that multiple sites or a particular configuration created by the 31-aa spacer in YadApstb may determine its uptake function.
The YadApstb Uptake Region Affects Hem- and Autoagglutination.
Because YadA surface appendages produce mannose-resistant hemagglutination of guinea pig erythrocytes and mediate bacterial agglutination (9, 28), we also tested whether a deletion of the uptake region of YadApstb had an influence on these processes (Fig. 6, which is published as supporting information on the PNAS web site). Rapid hemagglutination of erythrocytes was found with bacteria expressing the YadApstb protein, but was not induced by YadAent and YadApstbΔ53–83. Furthermore, when dense overnight cultures were prepared, it was observed that bacteria expressing YadApstb formed cloudy layers of biofilms on the glass tubes and settled out of suspension very rapidly. Biofilm formation and rapid aggregation were not observed with bacteria expressing YadAent or YadApstbΔ53–83. We conclude that the presence of the 31-aa uptake region is not only crucial for cell entry, but also determines the aggregation behavior of the bacteria.
In contrast, all YadA variants still conferred resistance against the bacteriolytic effect of human serum and induced identical levels of IL-8 production by epithelial cells (data not shown). This result was consistent with previous data (11), showing that the head and neck domain of YadA is dispensable for serum resistance and confirmed our recent study demonstrating that YadA-mediated cell adhesion, but not invasion, is required for IL-8 synthesis (29).
The YadA Derivatives Exhibited Different Binding Properties to Fibronectin, Collagen, and Laminin.
YadA has the capacity to interact with various ECM proteins, such as fibronectin, laminin, and collagen (20, 21) and induces cellular uptake through ECM-bound β1 integrin receptors (6). For this reason, differences in uptake could be based on different YadA-binding activities to ECM molecules. To address this question, we analyzed the ability of the YadA-expressing bacteria to bind to immobilized collagen, laminin, and fibronectin. The entry-defective YadAent and YadApstbΔ53–83 derivatives exhibited a similar, very strong capacity to bind collagen type I (Fig. 3A). YadApstb also mediated bacterial attachment to collagen, yet at levels 4- to 5-fold lower than YadAent and YadApstbΔ53–83 (P < 0.01). The identical binding pattern was found with collagen type II and IV, although the overall affinity of the YadA derivatives was ≈1.3-fold higher to collagen type II and ≈3-fold lower to collagen type IV (data not shown). The capacity of the YadA derivatives to interact with laminin was comparable with collagen. Only the noninvasive derivatives YadAent and YadApstbΔ53–83 conferred a high efficiency of laminin binding, whereas a much weaker interaction was detected with the invasion-efficient YadApstb protein (P < 0.01) (Fig. 3B). The interaction of YadA with immobilized fibronectin was generally lower than with collagen and laminin, but consistently above the background level. To quantitatively evaluate the ability of YadA to promote bacterial attachment to immobilized cellular fibronectin, we used a more sensitive method and counted stably bound bacteria by microscopic observation of stained samples. As shown in Fig. 3C, the YadApstb protein was able to efficiently mediate interaction with cellular fibronectin, whereas fibronectin binding via the YadAent and the YadApstbΔ53–83 proteins was low. Apparently, high binding efficiencies of YadA to collagen and laminin are accompanied by a strong reduction in fibronectin binding and vice versa. The ECM-binding properties are comparable in E. coli and Yersinia and demonstrate that the loss of the uptake domain in YadApstb induces a conversion of its specificity of ECM substrate binding.
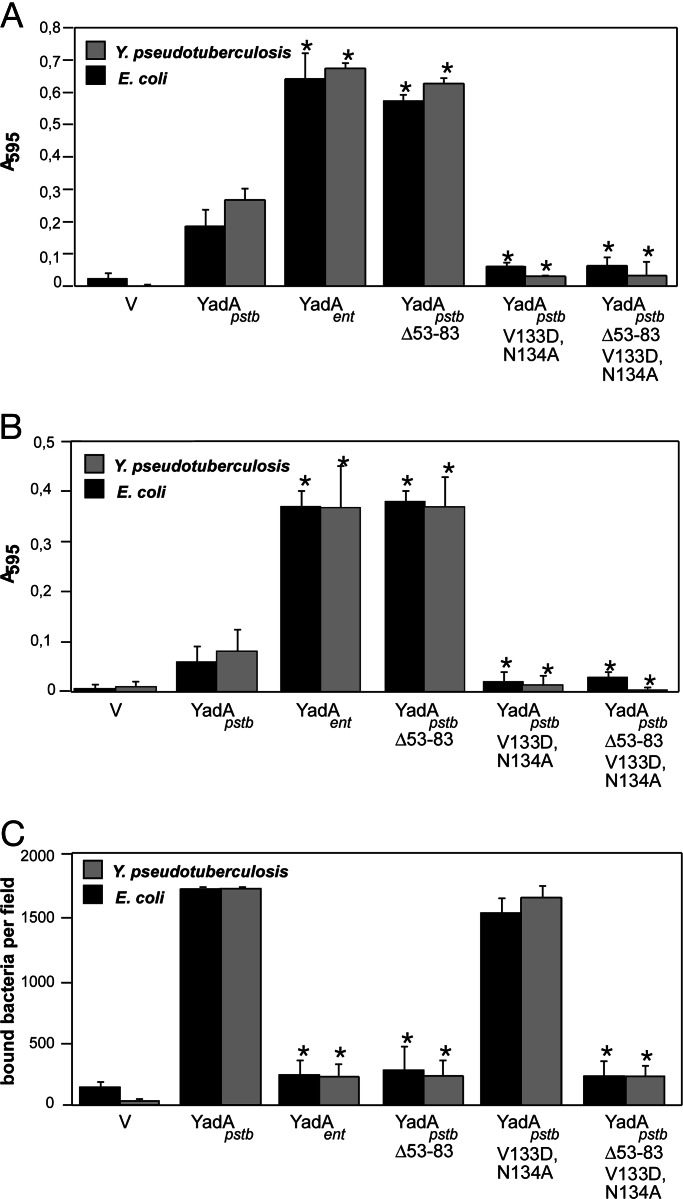
Fig. 3.
Different binding properties of YadA-expressing E. coli and Y. pseudotuberculosis YP31 strains to ECM molecules. Plastic wells were coated with collagen type I (A) or laminin (B). Bound bacteria were stained, and absorbance was measured at 595 nm. Expression of YadApstb results in a moderate interaction, whereas bacteria expressing YadAent and YadApstbΔ53–83 exhibit a strong binding capacity to collagen and laminin. Data represent the mean ± SD of three independent experiments done in duplicate. For quantitative analysis of adherence to fibronectin (C), glass coverslips were coated with cellular fibronectin and bound bacteria were counted in a defined area of a microscopic field of ≈4 × 103 μm2. The means and SD of 30 calculated fields are shown. Data were analyzed by the Student t test. ∗, significantly different from YadApstb-expressing bacteria (P < 0.05).
To gain more information about the connection between the individual ECM binding sites, we introduced a double mutation V133D/N134A into YadApstb and YadApstbΔ53–83, which was known to abrogate collagen binding of YadAent (12). As expected, the mutation fully abrogated collagen binding of YadApstbΔ53–83-expressing bacteria, but it also eliminated laminin binding and attachment to human epithelial cells (Figs. 1 and 3). In contrast, the YadApstbV133D/N134A derivative still mediated tight binding to cellular fibronectin and promoted cell adhesion and invasion similar to the WT YadApstb protein (Figs. 1 and 3). This result indicated that the binding regions for collagen and laminin may be linked but are distinct from the fibronectin-binding domain in the YadA head structure.
YadApstb-Mediated Uptake into HEp-2 Cells Mainly Occurs Through Fibronectin-Bound α5β1 Integrins.
Collagen, laminin, and fibronectin are bound to distinct β1 integrin receptors, i.e., α1β1, α2β1 by collagen, α3β1 by laminin, and αvβ1, α5β1 by fibronectin (30). Thus, it is possible that tight interaction of YadAent and YadApstbΔ53–83 with collagen and/or laminin might confer an adherent, but noninvasive, phenotype by sequestering the bacteria to invasion-incompetent β1 integrin receptors. To address this hypothesis, we preincubated HEp-2 cell monolayers with mAbs specific for fibronectin, laminin, or collagen type I and IV, which are predominantly expressed by epithelial cells, to selectively prevent ECM-mediated interaction with specific β1 integrins. Fig. 4 illustrates that, overall, YadA-mediated cell invasion was not significantly affected in the presence of different collagen type I and IV or laminin-specific antibodies. In contrast, antibodies directed against fibronectin strongly reduced bacterial internalization by YadApstb (P < 0.01) (Fig. 4), although overall adhesion to epithelial cells remained unaffected (data not shown). Thus, blockage of collagen and laminin binding alone does not seem to be sufficient to regenerate or enhance cell uptake of YadApstbΔ53–83 to YadApstb WT level. It rather seems that efficient invasion through YadA requires an appropriate interaction with fibronectin-bound β1 integrin receptors.
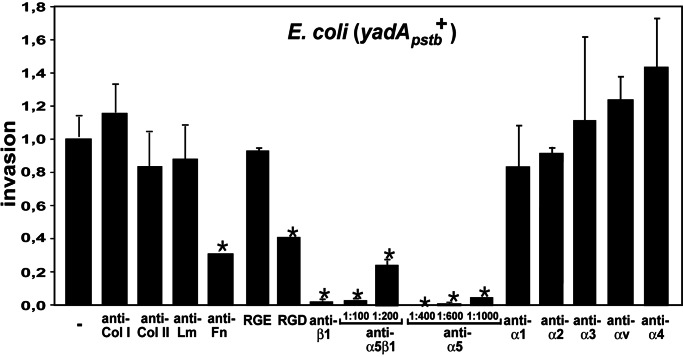
Fig. 4.
Inhibition of YadA-promoted invasion by blockage of fibronectin and the fibronectin-binding class of β1 integrins. HEp-2 cells were blocked with different antibodies directed against ECM molecules (Col, collagen; Lm, Laminin; Fn, fibronectin), GRGDSP/GRGESP peptides, and with antibodies to different α-subunits of β1 integrins before infection with YadA-expressing bacteria. Approximately 106 bacteria were used to challenge 5 × 104 cells, which were incubated for 1 h at 37°C to determine cell uptake efficiency. Only blockage of fibronectin and α5β1 integrins reduced YadA uptake. The proportionate reduction in invasion is expressed relative to the invasion rate promoted by the YadApstb WT protein, defined as 1.0. Each value represents the mean of three independent assays done in triplicate. Data were analyzed by the Student t test. ∗, P < 0.05 relative to YadApstb without antibodies/peptides (−).
Four different types of β1 integrin receptors have been observed to bind fibronectin: α4β1, α5β1, α8β1, and αvβ1, of which α5β1 is predominantly expressed on epithelial cells, whereas the others are less abundant and/or restricted to other cell types (30). All these integrins recognize a small amino acid motif, RGD, on fibronectin, whereas binding of collagen type I and IV to integrin α2β1 is independent of an RGD sequence (30, 31). Preincubation of HEp-2 cells with synthetic function-blocking GRGDSP peptides or α5/α5β1-specific antibodies (BIIG2, JBS5) strongly inhibited cellular invasion of YadApstb-expressing bacteria (P < 0.01), whereas GRGESP control peptides and function-blocking antibodies directed against the α1, α2, α3, αv, and α4 integrin subunits had no effect on YadApstb-mediated cell internalization (Fig. 4). This finding demonstrated that fibronectin-bound, but not collagen- or laminin-bound, integrin receptors are implicated in the YadA-promoted invasion process.
Effect of Invasion-Defective YadA Derivatives on Virulence.
To analyze whether differences in the invasion, aggregation, and ECM-binding properties of YadA have an effect on the Yersinia infection process, we compared the mouse infection potential of isogenic Y. pseudotuberculosis strains YP45 (yadA+), YP46 (yadAΔ53–83), and YP47 (yadA−) 2 days after infection. Strain YP45 colonized the Peyer’s patches and disseminated to mesenterial lymph nodes, spleen, and liver. In agreement with previous studies (32), the YadA-negative strain YP47 retained the ability to colonize host tissue, most likely due to other colonization factors, but considerably lower numbers of bacteria were detected in the Peyer’s patches, mesenterial lymph nodes, and spleen (Table 1). A similar difference in colonization of host tissue was observed between YP45 (yadA+) and the mutant strain YP46 (yadApstbΔ53–83), indicating that loss of the YadA invasion and/or aggregation function affects dissemination and attenuates the infection process.
Table 1.
Numbers of bacteria per organ on day 2 in Balb/c mice after orogastric infection
| Strain | PP | MLN | Spleen |
|---|---|---|---|
| YP45 (yadA+) | 2.1 × 108 ± 0.7 × 108 | 3.8 × 108 ± 0.1 × 108 | 5.3 × 105 ± 0.6 × 105 |
| YP46 (yadA Δ53–83) | 3.0 × 106 ± 0.2 × 106* | 4.6 × 106 ± 1.1 × 106* | 5.3 × 104 ± 1.3 × 104* |
| YP47 (yadA−) | 3.4 × 106 ± 0.2 × 106* | 6.9 × 106 ± 2.6 × 106* | 5.9 × 104 ± 0.4 × 104* |
Two groups of eight Balb/c mice were infected with 5 × 109 bacteria at day 0. At day 2, the numbers of colony-forming bacteria (cfu/g of tissue) in the Peyer’s Patches (PP), the mesenterial lymph nodes (MLN), and the spleen were determined by plating. Data were analyzed by the Student t test; ∗, P < 0.05 versus strain YP45.
Discussion
Virulence properties of microbial pathogens generally include the ability to adhere to different cell surface components. To accomplish cell adhesion, enteropathogenic Yersinia species express a multifunctional afimbrial adhesin, YadA, that shares many structural characteristics with a class of trimeric outer membrane proteins identified in a number of proteobacterial pathogens (2, 13, 15). The YadA proteins of enteric Yersinia species are conserved and exhibit high sequence homologies; however, our data demonstrate that their virulence properties can be fundamentally different.
In line with previous data (7, 8, 23), we found that YadA of Y. enterocolitica and Y. pseudotuberculosis promote equal levels of cell adhesion. However, only YadApstb is highly efficient in mediating cell entry, whereas even high quantities of YadAent can only poorly induce uptake into epithelial cells. We identified an additional sequence motif of 31 aa in the YadApstb protein, and show that this region constitutes the uptake domain. The presence of this motif also enhances the potential of YadApstb to aggregate red blood cells and to induce autoagglutination and biofilm formation. The 31-aa uptake sequence resides in the N-terminal region of the YadA head structure that was previously shown to be implicated in cell attachment and the aggregation of bacteria in stationary cultures (9, 16). In EM studies, the head domains of individual YadAent trimers were seen interacting with each other in a zipper-like fashion (10). The additional 31 aa in the YadApstb head structure might facilitate this interaction and improve the aggregation behavior of the bacteria. The most striking observations are that YadApstb and YadAent have opposite ECM adhesion properties and that simply the loss of the 31-aa uptake motif in YadApstb (YadApstbΔ53–83) can induce a conversion in the activity of ECM substrate binding, which is identical to YadAent. How can such a transition of ECM-binding properties occur? Detailed information about the ECM-binding sites in YadA is still missing. However, Skurnik and coworkers (12, 20, 22) showed that collagen binding of YadAent is very sensitive to conformational changes and requires a particular 3D lock-nut structure of the trimeric YadA molecule, which is constituted of hydrophobic regions containing NSVAIG-S motif repeats in the C-terminal part of the head domain. The collagen fibril seems to make contact with several surface-exposed residues from two YadAent monomers (12). However, mutations in these residues (e.g., V98D/N99A), eliminating collagen binding of YadAent and YadApstbΔ53–83, do not impair fibronectin binding and cellular invasion of YadApstb, indicating that separate sites are responsible for these adhesion functions. Due to these observations, we propose that the 31-aa spacer creates an additional functional domain in the YadApstb head structure, which participates in fibronectin binding, agglutination, and invasion, whereas elements that constitute the high-affinity collagen- and laminin-binding regions in YadAent and YadApstbΔ53–83 are less accessible or exhibit a different nonfunctional conformation in YadApstb. Recently, two other homologous YadA-like adhesins, VompA and C, were identified of Bartonella quintana, which also exhibited different collagen binding activities (15), implying that this class of adhesion factors is highly variable in its cell adhesion properties.
Research on bacterial invasion is still drawn by the unsolved question: What differentiates bacterial adhesion and intracellular uptake? Here, we show that YadA-mediated invasion can be dissected from adhesion by an internal deletion, which is accompanied by a transition of the adhesin’s activity of ECM substrate binding. This result suggests that the binding strength to a particular ECM molecule and the quality of the ECM-bound host cell integrin receptor is crucial for YadA-promoted uptake. Obviously, binding to fibronectin-bound β1 integrin receptors is essential for cell entry. This finding is supported by the fact that inhibitors of fibronectin and fibronectin-specific α5β1 receptors, but not blocking of other ECM molecules and integrins, impaired YadApstb-mediated cell invasion. Neither high-affinity binding to collagen nor laminin of YadAent and YadApstbΔ53–83 triggers invasion, indicating that collagen- and laminin-bound β1 integrin receptors are not, or are much less, competent in promoting uptake. In line with this assumption, a general impact on cell invasion has primarily been reported for the fibronectin-binding class of integrin receptors (33–36). Accordingly, weak collagen- and laminin-binding activity of YadApstb could also be advantageous for the invasion process, because bacteria are not captured by incompetent receptor complexes.
Several lines of evidence indicate that simple attachment to fibronectin-bound integrins is required, but it seems that a certain binding strength is also needed for initiating the entry process. Our data show that YadAent and YadApstbΔ53–83 are invasion-deficient, but they still interact with fibronectin, even though their binding capacity is low when compared with YadApstb. Also the noninvasive YadApstbA58E and K59A mutants still bind fibronectin (T.H., unpublished results) and adhere to epithelial cells, yet they are only poorly internalized, similar to fibronectin-coated latex beads (37). It has been reported that cooperative binding of the adhesin SfbI with two adjacent sites on human fibronectin is important for invasion of Streptococcus pyogenes into human epithelial cells (38). Accordingly, YadApstb may possess additional target sites for fibronectin, due to the 31-aa spacer. Alternatively, residues within the uptake domain of YadApstb might act as trigger factors, generating a conformational change in the YadA-fibronectin–β1 integrin complex that stabilizes YadA–fibronectin interaction and induces bacterial phagocytosis.
It is unclear what pressures resulted in Y. pseudotuberculosis and Y. enterocolitica having YadA molecules with different invasion, ECM binding, and aggregation properties. Most likely, these differences have consequences on the relative courses of infection taken by these bacteria. (i) In the oral mouse infection model, we detected considerably less bacteria in gut-associated tissues and organs with the Y. pseudotuberculosis yadApstbΔ53–83 mutant, with respect to an isogenic yadA+ strain. This finding implies that YadApstb fosters more efficient colonization of host tissues, probably due to better penetration of the intestinal layer. The Y. pseudotuberculosis yadA+ strain YP45 exhibited a much lower collagen-binding activity (T.H., unpublished results), but this result did not seem to affect colonization of the mesenterial lymph nodes, liver, and spleen. This result was unexpected, because it has been assumed that YadA-mediated collagen binding is important for colonization, because collagen-binding negative yadA mutant strains of Y. enterocolitica fail to disseminate from the Peyer’s Patches and are completely avirulent in mice (20, 39, 40). (ii) Due to differences in their YadA adhesion properties, Y. pseudotuberculosis and Y. enterocolitica might be directed to different tissue sites and may cause distinct colonization patterns in host tissues after the translocation process. Y. enterocolitica generally occurs in extracellular deeper tissue sites and forms abscesses including many bacteria (41). In Y. pseudotuberculosis infections, the bacteria are often visible as discrete microcolonies scattered in the tissues, and only few abscesses are found. Although most bacteria seem to be extracellular, it could not be ruled out that rare bacteria do multiply intracellularly (42).
In summary, we have demonstrated that different virulence properties are attributable to the highly similar YadA proteins of enteropathogenic Yersiniae. We identified a unique motif in the YadApstb head region that determines the specificity of ECM substrate binding, enhances hem- and autoaggregation, and triggers cell invasion via fibronectin-bound β1 integrin receptors. These properties foster a more efficient colonization of host tissues. We conclude that even small variations in individual virulence factors could define major differences in the pathogenicity of related pathogens.
Materials and Methods
Bacterial Strains, Cell Culture, and Media.
Bacterial strains (Table 2, which is published as supporting information on the PNAS web site) and HEp-2 cells were cultured as described (6).
DNA Manipulations and Plasmid and Strain Constructions.
Preparations of plasmid DNA, restriction digestions, ligations, transformations, and PCRs were performed as described (43). Plasmids (Table 2) harboring the different yadA alleles were constructed by inserting PCR-derived fragments, encoding the entire yadA gene of Y. pseudotuberculosis serotype III (YPIII) or Y. enterocolitica 8081v serotype O:8, into the XbaI and EcoRI sites of pBAD18 (44). Internal deletions and site-specific mutagenesis to introduce alternative amino acids in YadA were performed by a four-primer/two-step PCR procedure of pPD284 (yadA+) (6) with two common external and two internal mutagenesis primers. The resulting fragments encoding the 5′ end of the yadA gene with desired mutations were cloned into the KpnI/PvuII sites of pPD284 and confirmed by DNA sequencing. For construction of Y. pseudotuberculosis strains YP45 and YP46, the yadApstb WT gene and the yadApstbΔ53–83 alleles were cloned onto pGP704::Amp and mobilized into Y. pseudotuberculosis YP47, harboring a yadApstb knock-out mutation on pIB1. Proper integration into the yadA locus on the virulence plasmid of the transconjugants was analyzed by PCR, and expression of the YadApstb and the YadApstbΔ53–83 proteins was tested as described below.
YadA Protein Expression in the Outer Membrane of E. coli and Y. pseudotuberculosis.
To compare surface expression of the YadA proteins, whole-cell extracts of YadA-expressing bacteria were prepared, and total membranes were separated by SDS/PAGE and detected as described (6).
Adhesion and Invasion Assays.
Adhesion and invasion assays of E. coli and Y. pseudotuberculosis expressing the different YadA derivatives to HEp-2 cells were performed and quantified as described (6). For the inhibition experiments, anti-Fn antibodies (Calbiochem and Sigma), anti-collagen type I, II, and IV antibodies (Chemicon and Sigma), anti-laminin antibodies (Chemicon), GRGDSP/GRGESP peptides (GIBCO), and monoclonal integrin-blocking antibodies against α1 5E8D9 (Upstate Biotechnology, Lake Placid, NY), α2 P1E6 (Chemicon), α3 P1B5 (Chemicon), α4 P1H4 (Chemicon), α5 BIIG2 (Hybridoma Bank, University of Iowa), α5β1 (Chemicon), αv M9 (Chemicon), and β1 4B7 (Calbiochem) were diluted, and antibody solutions were incubated with the HEp-2 cell monolayers for 30 min at room temperature before addition of the bacteria.
ECM Binding.
To analyze the ability of the YadA proteins to interact with ECM proteins, 96-well Immuno Maxisorb plates (NUNC) were coated with 10 μg/ml collagen type I, II, or IV or laminin (Sigma) in PBS at 4°C overnight, washed with TBS (150 mM NaCl/20 mM Tris·HCl, pH 7.5) and blocked with TBS plus 2% BSA (Sigma) for 1 h. Overnight cultures of YadA-expressing bacteria were washed with TBS, and equal amounts (106) were added to the ECM protein-coated wells. TBS alone and E. coli harboring vector pBAD18 were used as controls. After incubation for 2 h, nonbound bacteria were removed, and adherent bacteria were fixed with 4% paraformaldehyde for 20 min and stained with crystal violet for 10 min. After washing with TBS, stained bacteria were detected at OD450 with a microplate reader Model 550 (Bio-Rad). To analyze fibronectin binding, 10 μg/ml cellular fibronectin (Sigma) in PBS was used to coat cover slips in a 24-well plate at 4°C overnight. Cover slips were then blocked with PBS plus 2% BSA, and 107 YadA-expressing bacteria in PBS plus 2% BSA were inoculated into each fibronectin-coated well and incubated at 37°C for 1 h. After washing, bacteria were fixed with 4% paraformaldehyde for 20 min and stained with crystal violet. Bound bacteria were mounted in PBS containing 80% glycerol, and 30 representative fields of three independent samples were counted by using a Zeiss Axioskope II.
Serum Resistance Experiments.
Human serum was used to determine serum resistance mediated by the YadA proteins. The serum was incubated at 56°C for 30 min to inactivate the complement system as a control. Ten microliters of a suspension of 300–600 YadA-expressing bacteria were incubated at 37°C in 5% normal or heat-inactivated serum for 1 h. Surviving bacteria were determined by plating out serial dilutions to determine the colony-forming units. The bactericidal effect of the serum was calculated as the survival relative to the survival of bacteria incubated in inactivated serum.
Autoagglutination and Hemagglutination Experiments.
To test autoagglutination, overnight cultures of YadA-expressing bacteria were incubated in glass tubes at room temperature without shaking. Rapid clearance of the medium, aggregated bacteria at the bottom, and biofilm at the glass tube were recorded with a digital camera (Sony). To quantify autoagglutination, the overnight cultures were diluted in fresh medium to obtain an OD600 of 1.0, and autoagglutination was measured as the decrease in the optical density over time. For the hemagglutination assay, overnight cultures of YadA-expressing bacteria were adjusted to an OD600 of 0.3, and serial 2-fold dilutions of this suspension were mixed with equal amounts of a 2% (vol/vol) suspension of sheep erythrocytes (Oxoid, Basingstoke, U.K.) in PBS in a 96-well U-bottom polypropylene plate (Costar). The microtiter plate was agitated on a mixer for 30 s. Hemagglutination was recorded photographically after 15 min.
Virulence Experiments.
Groups of eight BALB/c mice (6–8 weeks, female) were orally infected with 5 × 109 bacteria of Y. pseudotuberculosis strains YP45, YP46, or YP47 (animal licensing committee permission no. G0194/00). After 2 days of infection, mice were killed, and the organs were homogenized in PBS. The ileum was incubated for 30 min with 100 μg/ml gentamicin and then intensively washed with PBS. The numbers of bacteria were determined by plating three independent serial dilutions of the homogenates on Yersinia selective medium (Oxoid).
Supplementary Material
Acknowledgments
We thank Drs. Birgitta Beatrix, Martin Fenner, Abigail Mavor, Eckhard Strauch, and Kursad Turgay for helpful discussion and critical reading of the manuscript and all laboratory members for technical support. This work was supported by Deutsche Forschungsgemeinschaft Grant DE616/2.
Abbreviations
- ECM
extracellular matrix.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bottone E. J. Clin. Microbiol. Rev. 1997;10:257–276. doi: 10.1128/cmr.10.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pepe J. C., Wachtel M. R., Wagar E., Miller V. L. Infect. Immun. 1995;63:4837–4848. doi: 10.1128/iai.63.12.4837-4848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marra A., Isberg R. R. Infect. Immun. 1997;65:3412–3421. doi: 10.1128/iai.65.8.3412-3421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leong J. M., Fournier R. S., Isberg R. R. EMBO J. 1990;9:1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark M. A., Hirst B. H., Jepson M. A. Infect. Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eitel J., Dersch P. Infect. Immun. 2002;70:4880–4891. doi: 10.1128/IAI.70.9.4880-4891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y., Isberg R. R. Infect. Immun. 1993;61:3907–3913. doi: 10.1128/iai.61.9.3907-3913.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bliska J. B., Copass M. C., Falkow S. Infect. Immun. 1993;61:3914–3921. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Tahir Y., Skurnik M. Int. J. Med. Microbiol. 2001;291:209–218. doi: 10.1078/1438-4221-00119. [DOI] [PubMed] [Google Scholar]
- 10.Hoiczyk E., Roggenkamp A., Reichenbecher M., Lupas A., Heesemann J. EMBO J. 2000;19:5989–5999. doi: 10.1093/emboj/19.22.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roggenkamp A., Ackermann N., Jacobi C. A., Truelzsch K., Hoffmann H., Heesemann J. J. Bacteriol. 2003;185:3735–3744. doi: 10.1128/JB.185.13.3735-3744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nummelin H., Merckel M. C., Leo J. C., Lankinen H., Skurnik M., Goldman A. EMBO J. 2004;23:701–711. doi: 10.1038/sj.emboj.7600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comanducci M., Bambini S., Brunelli B., Adu-Bobie J., Arico B., Capecchi B., Giuliani M. M., Masignani V., Santini L., Savino S., et al. J. Exp. Med. 2002;195:1445–1454. doi: 10.1084/jem.20020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray S. K., Rajeshwari R., Sharma Y., Sonti R. V. Mol. Microbiol. 2002;46:637–647. doi: 10.1046/j.1365-2958.2002.03188.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang P., Chomel B. B., Schau M. K., Goo J. S., Droz S., Kelminson K. L., George S. S., Lerche N. W., Koehler J. E. Proc. Natl. Acad. Sci. USA. 2004;101:13630–13635. doi: 10.1073/pnas.0405284101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roggenkamp A., Ruckdeschel K., Leitritz L., Schmitt R., Heesemann J. Infect. Immun. 1996;64:2506–2514. doi: 10.1128/iai.64.7.2506-2514.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balligand G., Laroche Y., Cornelis G. Infect. Immun. 1985;48:782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapperud G., Namork E., Skarpeid H. J. Infect. Immun. 1985;47:561–566. doi: 10.1128/iai.47.2.561-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tertti R., Skurnik M., Vartio T., Kuusela P. Infect. Immun. 1992;60:3021–3024. doi: 10.1128/iai.60.7.3021-3024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamm A., Tarkkanen A. M., Korhonen T. K., Kuusela P., Toivanen P., Skurnik M. Mol. Microbiol. 1993;10:995–1011. doi: 10.1111/j.1365-2958.1993.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 21.Roggenkamp A., Neuberger H. R., Flugel A., Schmoll T., Heesemann J. Mol. Microbiol. 1995;16:1207–1219. doi: 10.1111/j.1365-2958.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 22.El Tahir Y. E., Kuusela P., Skurnik M. Mol. Microbiol. 2000;37:192–206. doi: 10.1046/j.1365-2958.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 23.Heesemann J., Gruter L. FEBS Microbiol. Lett. 1987;40:37–41. [Google Scholar]
- 24.Schmid Y., Grassl G. A., Buhler O. T., Skurnik M., Autenrieth I. B., Bohn E. Infect. Immun. 2004;72:6780–6789. doi: 10.1128/IAI.72.12.6780-6789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paerregaard A., Espersen F., Skurnik M. APMIS. 1991;99:226–232. doi: 10.1111/j.1699-0463.1991.tb05143.x. [DOI] [PubMed] [Google Scholar]
- 26.Leong J. M., Morrissey P. E., Marra A., Isberg R. R. EMBO J. 1995;14:422–431. doi: 10.1002/j.1460-2075.1995.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeng A., Sakota V., Li Z., Datta V., Beall B., Nizet V. J. Bacteriol. 2003;185:1208–1217. doi: 10.1128/JB.185.4.1208-1217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapperud G., Lassen J. Infect. Immun. 1983;42:163–169. doi: 10.1128/iai.42.1.163-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eitel J., Heise T., Thiesen U., Dersch P. Cell. Microbiol. 2005;7:63–77. doi: 10.1111/j.1462-5822.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 30.Eble J. A., Kühn K. Integrin-Ligand Interaction. Landes, Austin, TX: Springer; 1997. [Google Scholar]
- 31.Ruoslahti E. Annu. Rev. Cell Dev. Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 32.Han Y. W., Miller V. L. Infect. Immun. 1997;65:327–330. doi: 10.1128/iai.65.1.327-330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joh D., Wann E. R., Kreikemeyer B., Speziale P., Hook M. Matrix Biol. 1999;18:211–223. doi: 10.1016/s0945-053x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 34.Agerer F., Michel A., Ohlsen K., Hauck C. R. J. Biol. Chem. 2003;278:42524–42531. doi: 10.1074/jbc.M302096200. [DOI] [PubMed] [Google Scholar]
- 35.Watarai M., Funato S., Sasakawa C. J. Exp. Med. 1996;183:991–999. doi: 10.1084/jem.183.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virji M., Makepeace K., Moxon E. R. Mol. Microbiol. 1994;14:173–184. doi: 10.1111/j.1365-2958.1994.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 37.Hook M., Switalski L., Wastrom T., Lindeberg M. In: Fibronectin. Mosher D. F., editor. San Diego: Academic; 1989. pp. 295–308. [Google Scholar]
- 38.Talay S. R., Zock A., Rohde M., Molinari G., Oggioni M., Pozzi G., Guzman C. A., Chhatwal G. S. Cell Microbiol. 2000;2:521–535. doi: 10.1046/j.1462-5822.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 39.Gripenberg-Lerche C., Skurnik M., Zhang L., Soderstrom K. O., Toivanen P. Infect. Immun. 1994;62:5568–5575. doi: 10.1128/iai.62.12.5568-5575.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gripenberg-Lerche C., Skurnik M., Toivanen P. Infect. Immun. 1995;63:3222–3226. doi: 10.1128/iai.63.8.3222-3226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Une T. Microbiol. Immunol. 1977;21:341–363. [PubMed] [Google Scholar]
- 42.Simonet M., Richard S., Berche P. Infect. Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook J. Molecular Cloning: A Laboratory Manual. Woodbury, NY: Cold Spring Harbor Lab. Press; 2001. [Google Scholar]
- 44.Guzman L. M., Belin D., Carson M. J., Beckwith J. J. Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.