Abstract
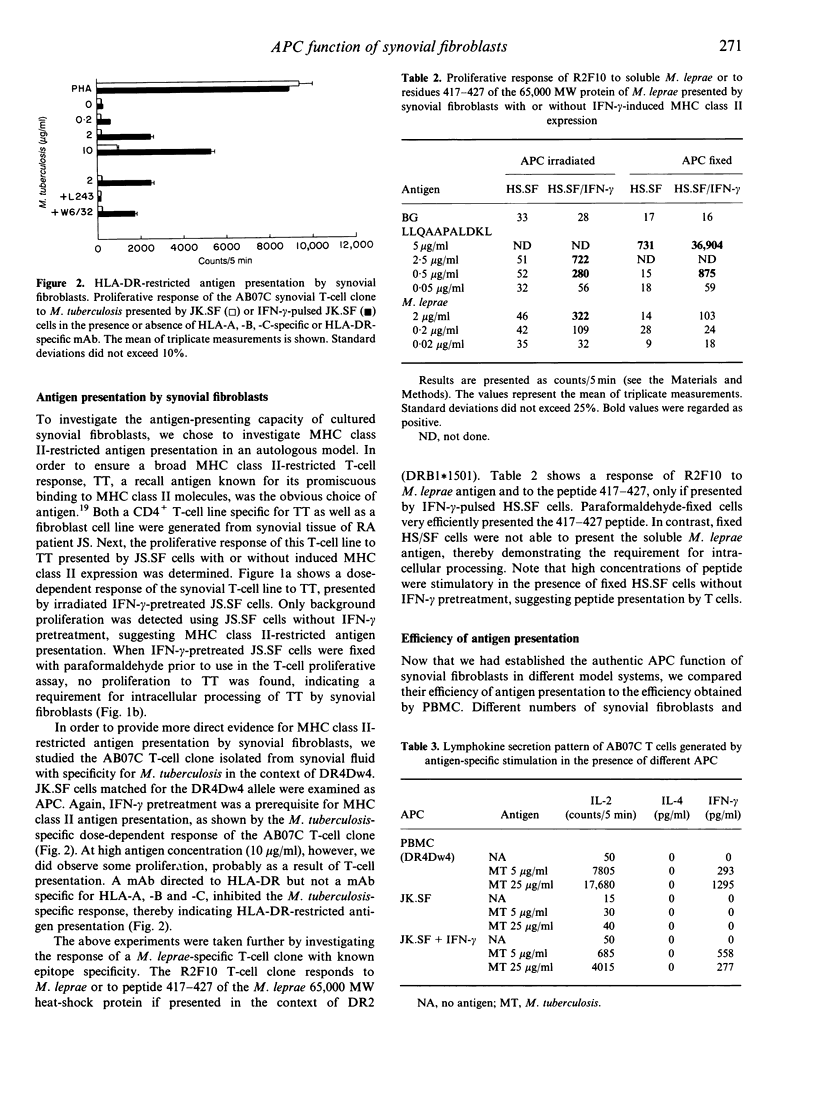
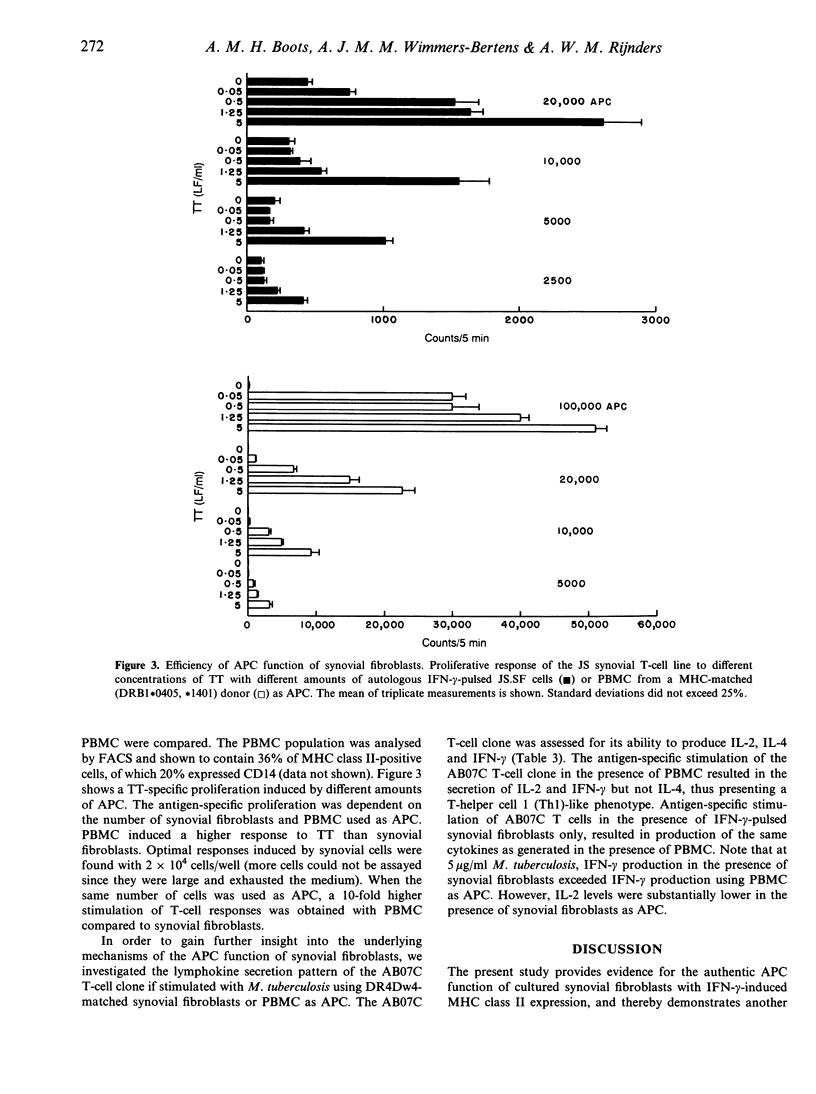
In normal, healthy joints, synovial fibroblasts do not express major histocompatibility complex (MHC) class II molecules. However, in inflamed joints of rheumatoid arthritis (RA) patients, synovial fibroblasts show an abundant expression of MHC class II. Does this increase in expression have functional consequences for antigen presentation to T cells? To date, the precise role of synovial fibroblasts in antigen presentation has not been documented. Here, we show by three different examples that cultured synovial fibroblasts with interferon-gamma (IFN-gamma)-induced MHC class II expression are capable of processing soluble protein for presentation to CD4+ T cells. First, the antigen-presenting cell (APC) function of synovial fibroblasts was studied in an autologous model. From synovial tissue of a RA patient both a fibroblast cell line and a tetanus toxoid (TT)-specific CD4+ T-cell line were generated. A dose-dependent TT response was observed only when TT was presented by IFN-gamma-pretreated synovial fibroblasts. As more direct evidence for MHC class II-restricted antigen presentation, the response of a Mycobacterium tuberculosis-specific CD4+ T-cell clone isolated from rheumatoid synovial fluid was demonstrated in the presence of synovial fibroblasts. The response was DR4Dw4-restricted and could be inhibited by monoclonal antibody (mAb) to HLA-DR. In addition, the lymphokine secretion pattern of the synovial T-cell clone did not differ qualitatively upon antigen-specific stimulation using peripheral blood mononuclear cells (PBMC) or synovial fibroblasts as APC. In order to provide evidence for intracellular antigen processing we next examined the response of a M. leprae-specific T-cell clone with known epitope specificity. Our data suggest that synovial fibroblasts are not passive bystanders, but can become active participants in the development and maintenance of chronic inflammation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvaro-Gracia J. M., Zvaifler N. J., Firestein G. S. Cytokines in chronic inflammatory arthritis. IV. Granulocyte/macrophage colony-stimulating factor-mediated induction of class II MHC antigen on human monocytes: a possible role in rheumatoid arthritis. J Exp Med. 1989 Sep 1;170(3):865–875. doi: 10.1084/jem.170.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amento E. P., Bhan A. K., McCullagh K. G., Krane S. M. Influences of gamma interferon on synovial fibroblast-like cells. Ia induction and inhibition of collagen synthesis. J Clin Invest. 1985 Aug;76(2):837–848. doi: 10.1172/JCI112041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., van Schooten W. C., Janson A., Barry M. E., de Vries R. R. Molecular mapping of interactions between a Mycobacterium leprae-specific T cell epitope, the restricting HLA-DR2 molecule, and two specific T cell receptors. J Immunol. 1990 Apr 1;144(7):2459–2464. [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bucala R., Ritchlin C., Winchester R., Cerami A. Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J Exp Med. 1991 Mar 1;173(3):569–574. doi: 10.1084/jem.173.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Rohwer P., Zacher J., Winchester R. J., Kalden J. R. Differential expression of Ia antigens by rheumatoid synovial lining cells. J Clin Invest. 1987 Sep;80(3):595–604. doi: 10.1172/JCI113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. E., Winterrowd G. E., Krzesicki R. F., Sanders M. E. Role of cytokines in inflammatory synovitis. The coordinate regulation of intercellular adhesion molecule 1 and HLA class I and class II antigens in rheumatoid synovial fibroblasts. Arthritis Rheum. 1990 Dec;33(12):1776–1786. doi: 10.1002/art.1780331204. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M., Littman B. H. Gene expression (collagenase, tissue inhibitor of metalloproteinases, complement, and HLA-DR) in rheumatoid arthritis and osteoarthritis synovium. Quantitative analysis and effect of intraarticular corticosteroids. Arthritis Rheum. 1991 Sep;34(9):1094–1105. doi: 10.1002/art.1780340905. [DOI] [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Antigen presentation by interferon-gamma-treated endothelial cells and fibroblasts: differential ability to function as antigen-presenting cells despite comparable Ia expression. J Immunol. 1985 Dec;135(6):3750–3762. [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Dissection of defective antigen presentation by interferon-gamma-treated fibroblasts. J Immunol. 1987 Jan 15;138(2):385–392. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Goto M., Sasano M., Yamanaka H., Miyasaka N., Kamatani N., Inoue K., Nishioka K., Miyamoto T. Spontaneous production of an interleukin 1-like factor by cloned rheumatoid synovial cells in long-term culture. J Clin Invest. 1987 Sep;80(3):786–796. doi: 10.1172/JCI113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Scheynius A., Kabelitz D., Wigzell H. Evidence in support of a self-perpetuating HLA-DR-dependent delayed-type cell reaction in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3632–3636. doi: 10.1073/pnas.79.11.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac Z., Schwartz R. H. The molecular basis of the requirement for antigen processing of pigeon cytochrome c prior to T cell activation. J Immunol. 1985 May;134(5):3233–3240. [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Life P. F., Viner N. J., Bacon P. A., Gaston J. S. Synovial fluid antigen-presenting cells unmask peripheral blood T cell responses to bacterial antigens in inflammatory arthritis. Clin Exp Immunol. 1990 Feb;79(2):189–194. doi: 10.1111/j.1365-2249.1990.tb05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Ducret J., Wayner E., Elices M. J., Alvaro-Gracia J. M., Zvaifler N. J., Firestein G. S. Alpha 4/beta 1 integrin (VLA-4) ligands in arthritis. Vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J Immunol. 1992 Aug 15;149(4):1424–1431. [PubMed] [Google Scholar]
- Mourad W., Mehindate K., Schall T. J., McColl S. R. Engagement of major histocompatibility complex class II molecules by superantigen induces inflammatory cytokine gene expression in human rheumatoid fibroblast-like synoviocytes. J Exp Med. 1992 Feb 1;175(2):613–616. doi: 10.1084/jem.175.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panina-Bordignon P., Tan A., Termijtelen A., Demotz S., Corradin G., Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989 Dec;19(12):2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Res P. C., Orsini D. L., Van Laar J. M., Janson A. A., Abou-Zeid C., De Vries R. R. Diversity in antigen recognition by Mycobacterium tuberculosis-reactive T cell clones from the synovial fluid of rheumatoid arthritis patients. Eur J Immunol. 1991 May;21(5):1297–1302. doi: 10.1002/eji.1830210530. [DOI] [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990 Feb;11(2):49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Wordsworth B. P., Allsopp C. E., Young R. P., Bell J. I. HLA-DR typing using DNA amplification by the polymerase chain reaction and sequential hybridization to sequence-specific oligonucleotide probes. Immunogenetics. 1990;32(6):413–418. doi: 10.1007/BF00241635. [DOI] [PubMed] [Google Scholar]


