Abstract
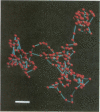
The spontaneous locomotion of immunomagnetically isolated resting CD4+ and CD8+ lymphocytes in three-dimensional collagen gels was recorded by time-lapse videomicroscopy. Two-dimensional projections of the paths of randomly selected individual cells were digitized, plotted and quantitatively analysed. Among five different donors 46 +/- 10% of CD4+ and CD8+ lymphocytes (n = 180) showed initial spontaneous locomotion (individual speed 5-25 microns/min; mean speed CD4+ 5.2 +/- 3.6 microns, CD8+ 3.23 +/- 2.72 microns). Active CD4+ cells were constantly migrating for more than 4 hr, whereas CD8+ lymphocytes significantly slowed down after 60-90 min in the collagen gel (P < 0.003). Quantitative analysis of the paths indicated at least three migratory phenotypes: (1) spontaneously locomoting cells exhibiting high speed and low frequency of stopping; (2) a major non-motile fraction without significant displacement; and (3) a subpopulation within CD4+ and CD8+ cells with intermediate activity of speed and stopping. Further subtype analysis of immunomagnetically isolated CD45RAhigh/ROlow or CD45RAlow/ROhigh lymphocytes showed that more than 90% of CD4+ CD45RAhigh/ROlow cells were actively locomoting. In contrast, only 20% of the CD4+ CD45ROhigh/RAlow phenotype showed spontaneous motility to a limited degree. The data indicate that resting CD4+ and CD8+ lymphocytes comprise further locomotory subpopulation related to the expression of different CD45 isoforms.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arencibia I., Sundqvist K. G. Collagen receptor on T lymphocytes and the control of lymphocyte motility. Eur J Immunol. 1989 May;19(5):929–934. doi: 10.1002/eji.1830190521. [DOI] [PubMed] [Google Scholar]
- Bell E. B., Sparshott S. M. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990 Nov 8;348(6297):163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Eberhardt C., Van der Vieren M. Direct interaction with primed CD4+ CD45R0+ memory T lymphocytes induces expression of endothelial leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1 on the surface of vascular endothelial cells. Eur J Immunol. 1991 Dec;21(12):2915–2923. doi: 10.1002/eji.1830211204. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Linsley P. S., Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J Immunol. 1992 Apr 1;148(7):1985–1992. [PubMed] [Google Scholar]
- Friedl P., Noble P. B., Zänker K. S. Lymphocyte locomotion in three-dimensional collagen gels. Comparison of three quantitative methods for analysing cell trajectories. J Immunol Methods. 1993 Oct 15;165(2):157–165. doi: 10.1016/0022-1759(93)90341-4. [DOI] [PubMed] [Google Scholar]
- Haston W. S., Shields J. M., Wilkinson P. C. Lymphocyte locomotion and attachment on two-dimensional surfaces and in three-dimensional matrices. J Cell Biol. 1982 Mar;92(3):747–752. doi: 10.1083/jcb.92.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkoviz R., Gilat D., Miron S., Mekori Y. A., Aderka D., Wallach D., Vlodavsky I., Cohen I. R., Lider O. Extracellular matrix induces tumour necrosis factor-alpha secretion by an interaction between resting rat CD4+ T cells and macrophages. Immunology. 1993 Jan;78(1):50–57. [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Asano Y., Matsuoka S., Furutani-Seiki M., Aizawa S., Nishimura H., Shirai T., Tada T. Distinction of mouse CD8+ suppressor effector T cell clones from cytotoxic T cell clones by cytokine production and CD45 isoforms. J Immunol. 1993 Mar 15;150(6):2121–2128. [PubMed] [Google Scholar]
- Koretzky G. A. Role of the CD45 tyrosine phosphatase in signal transduction in the immune system. FASEB J. 1993 Mar;7(5):420–426. doi: 10.1096/fasebj.7.5.8462784. [DOI] [PubMed] [Google Scholar]
- Li Y. Y., Cheung H. T. Basement membrane and its components on lymphocyte adhesion, migration, and proliferation. J Immunol. 1992 Nov 15;149(10):3174–3181. [PubMed] [Google Scholar]
- Mackay C. R., Marston W. L., Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990 Mar 1;171(3):801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Marston W. L., Dudler L., Spertini O., Tedder T. F., Hein W. R. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992 Apr;22(4):887–895. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- Mackay C. R. T-cell memory: the connection between function, phenotype and migration pathways. Immunol Today. 1991 Jun;12(6):189–192. doi: 10.1016/0167-5699(91)90051-T. [DOI] [PubMed] [Google Scholar]
- Masuyama J., Berman J. S., Cruikshank W. W., Morimoto C., Center D. M. Evidence for recent as well as long term activation of T cells migrating through endothelial cell monolayers in vitro. J Immunol. 1992 Mar 1;148(5):1367–1374. [PubMed] [Google Scholar]
- Newman I., Wilkinson P. C. Locomotor responses of human CD45 lymphocyte subsets: preferential locomotion of CD45RO+ lymphocytes in response to attractants and mitogens. Immunology. 1993 Jan;78(1):92–98. [PMC free article] [PubMed] [Google Scholar]
- Noble P. B. Extracellular matrix and cell migration: locomotory characteristics of MOS-11 cells within a three-dimensional hydrated collagen lattice. J Cell Sci. 1987 Mar;87(Pt 2):241–248. doi: 10.1242/jcs.87.2.241. [DOI] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Bogue D. T., Ramberg J., Lipsky P. E. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991 Nov 1;147(9):2913–2921. [PubMed] [Google Scholar]
- Picker L. J., Butcher E. C. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Treer J. R., Ferguson-Darnell B., Collins P. A., Buck D., Terstappen L. W. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selectin on T cells during the virgin to memory cell transition. J Immunol. 1993 Feb 1;150(3):1105–1121. [PubMed] [Google Scholar]
- Pietschmann P., Cush J. J., Lipsky P. E., Oppenheimer-Marks N. Identification of subsets of human T cells capable of enhanced transendothelial migration. J Immunol. 1992 Aug 15;149(4):1170–1178. [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Ratner S., Patrick P., Bora G. Lymphocyte development of adherence and motility in extracellular matrix during IL-2 stimulation. J Immunol. 1992 Jul 15;149(2):681–688. [PubMed] [Google Scholar]
- Saltini C., Kirby M., Trapnell B. C., Tamura N., Crystal R. G. Biased accumulation of T lymphocytes with "memory"-type CD45 leukocyte common antigen gene expression on the epithelial surface of the human lung. J Exp Med. 1990 Apr 1;171(4):1123–1140. doi: 10.1084/jem.171.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Shimizu Y., Shaw S., Graber N., Gopal T. V., Horgan K. J., Van Seventer G. A., Newman W. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1991 Feb 28;349(6312):799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S. Lymphocyte interactions with extracellular matrix. FASEB J. 1991 Jun;5(9):2292–2299. doi: 10.1096/fasebj.5.9.1860621. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol. 1990 Jul 1;145(1):59–67. [PubMed] [Google Scholar]
- Wagner N., Engel P., Tedder T. F. Regulation of the tyrosine kinase-dependent adhesion pathway in human lymphocytes through CD45. J Immunol. 1993 Jun 1;150(11):4887–4899. [PubMed] [Google Scholar]
- de Jong R., Brouwer M., Miedema F., van Lier R. A. Human CD8+ T lymphocytes can be divided into CD45RA+ and CD45RO+ cells with different requirements for activation and differentiation. J Immunol. 1991 Apr 1;146(7):2088–2094. [PubMed] [Google Scholar]