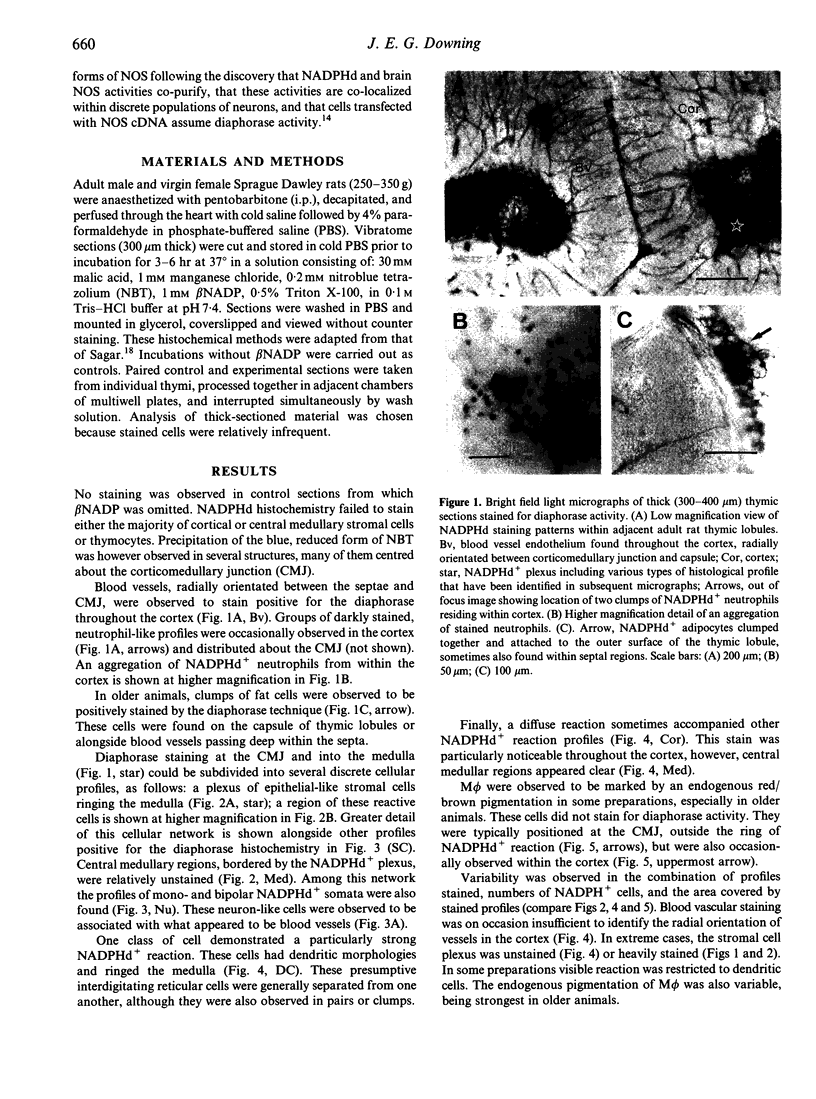
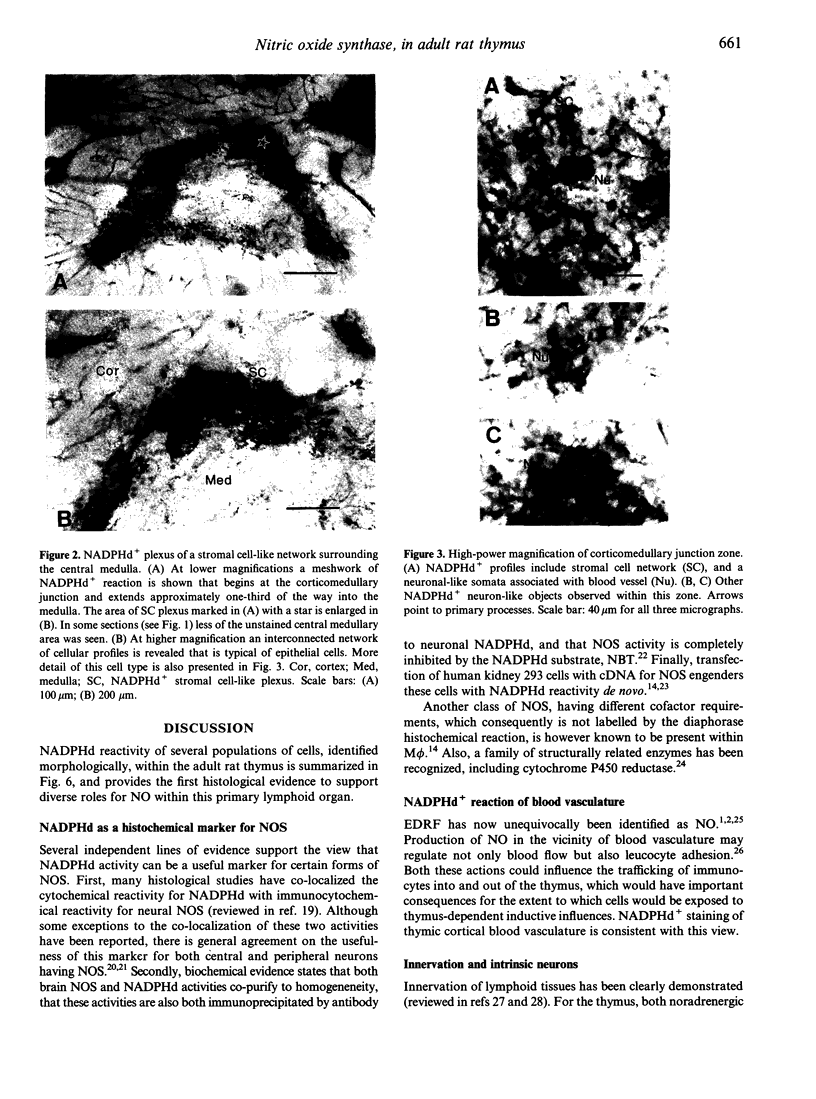
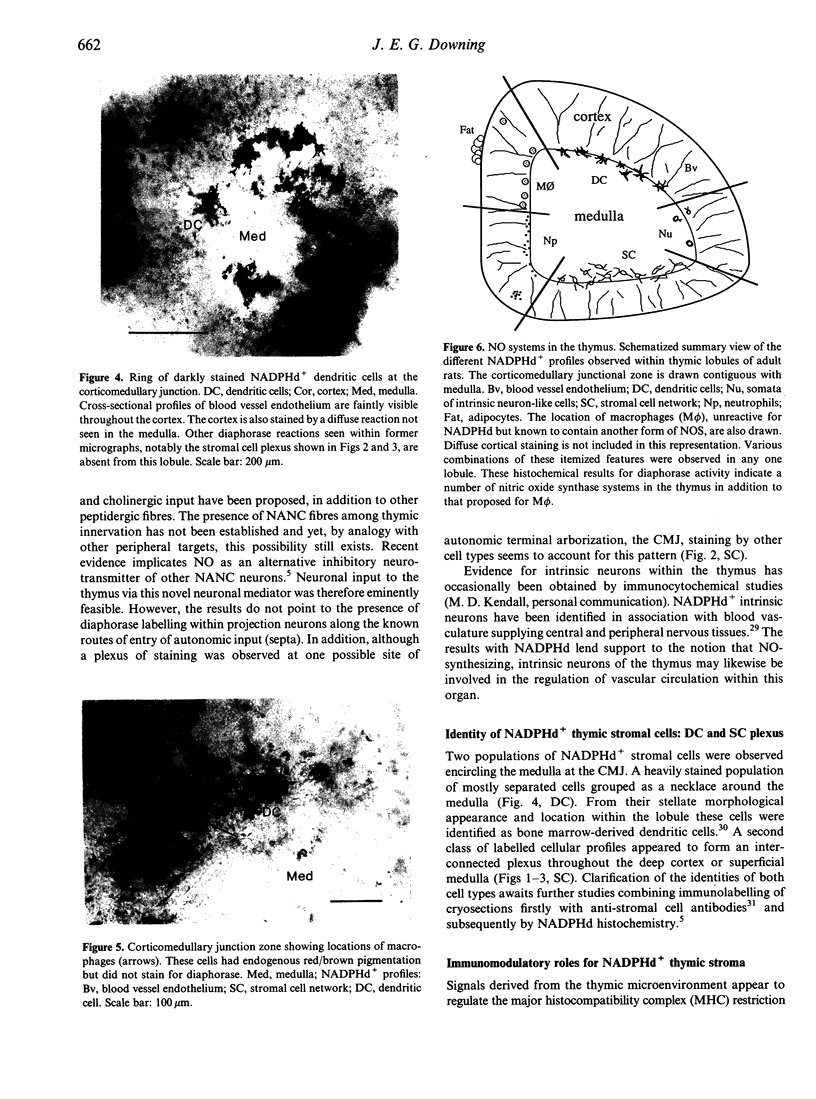
Abstract
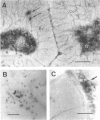
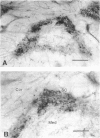
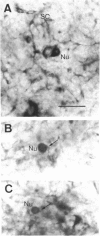
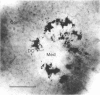
Nitric oxide (NO) has become recognized as a multifunctional mediator, with roles in vascular physiology, neurotransmission and non-specific immune defense. The histochemical marker associated with the neural and endothelial form of NO synthase (NOS), reduced nicotinamide adenine dinucleotide diaphorase (NADPHd), has enabled the indirect localization of potential sites of NO production. Innervation of the thymus and its immunological functions made this tissue a candidate for utilization of various NO systems. In the present study on adult rat thymus, multiple cellular sites expressing NADPHd activity, thereby implicated as sites of NOS activity, have been identified using morphological criteria alone: blood vessel endothelium, dendritic cells, deep cortical or medullary stromal cells, intrinsic neuron-like profiles, granulocytes (possibly neutrophils) and fat cells. In addition, the availability to the thymic microenvironment of another form of NOS in macrophages, which is not stained by the diaphorase technique, was supported by the observation of these cells at corticomedullary and cortical locations. These results indicate that a wide variety of possible immunomodulatory roles can be expected for NO in the thymus including the induction of tolerance, major histocompatibility complex (MHC) restriction, lymphocyte trafficking and regulation of thymic endocrine output.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ader R., Felten D., Cohen N. Interactions between the brain and the immune system. Annu Rev Pharmacol Toxicol. 1990;30:561–602. doi: 10.1146/annurev.pa.30.040190.003021. [DOI] [PubMed] [Google Scholar]
- Anderson G., Jenkinson E. J., Moore N. C., Owen J. J. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature. 1993 Mar 4;362(6415):70–73. doi: 10.1038/362070a0. [DOI] [PubMed] [Google Scholar]
- Benoist C., Mathis D. Positive selection of the T cell repertoire: where and when does it occur? Cell. 1989 Sep 22;58(6):1027–1033. doi: 10.1016/0092-8674(89)90501-1. [DOI] [PubMed] [Google Scholar]
- Boyd R. L., Tucek C. L., Godfrey D. I., Izon D. J., Wilson T. J., Davidson N. J., Bean A. G., Ladyman H. M., Ritter M. A., Hugo P. The thymic microenvironment. Immunol Today. 1993 Sep;14(9):445–459. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris N., Mocchegiani E., Muzzioli M., Piloni S. Recovery of age-related decline of thymic endocrine activity and PHA response by lysin-arginine combination. Int J Immunopharmacol. 1986;8(7):677–685. doi: 10.1016/0192-0561(86)90002-0. [DOI] [PubMed] [Google Scholar]
- Fabris N., Mocchegiani E., Muzzioli M., Provinciali M. Neuroendocrine-thymus interactions: perspectives for intervention in aging. Ann N Y Acad Sci. 1988;521:72–87. doi: 10.1111/j.1749-6632.1988.tb35266.x. [DOI] [PubMed] [Google Scholar]
- Felten D. L., Felten S. Y., Carlson S. L., Olschowka J. A., Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985 Aug;135(2 Suppl):755s–765s. [PubMed] [Google Scholar]
- Grozdanovic Z., Baumgarten H. G., Brüning G. Histochemistry of NADPH-diaphorase, a marker for neuronal nitric oxide synthase, in the peripheral autonomic nervous system of the mouse. Neuroscience. 1992;48(1):225–235. doi: 10.1016/0306-4522(92)90351-2. [DOI] [PubMed] [Google Scholar]
- Hiki K., Yui Y., Hattori R., Eizawa H., Kosuga K., Kawai C. Three regulation mechanisms of nitric oxide synthase. Eur J Pharmacol. 1991 Feb 25;206(2):163–164. doi: 10.1016/0922-4106(91)90026-e. [DOI] [PubMed] [Google Scholar]
- Hoffman R. A., Langrehr J. M., Billiar T. R., Curran R. D., Simmons R. L. Alloantigen-induced activation of rat splenocytes is regulated by the oxidative metabolism of L-arginine. J Immunol. 1990 Oct 1;145(7):2220–2226. [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Beitz A. J., Renno W., Xu X., Mayer B., Zhang F. Nitric oxide synthase-containing neural processes on large cerebral arteries and cerebral microvessels. Brain Res. 1993 Mar 19;606(1):148–155. doi: 10.1016/0006-8993(93)91583-e. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidori A., Lo Monaco A., Cappa M. A study of growth hormone release in man after oral administration of amino acids. Curr Med Res Opin. 1981;7(7):475–481. doi: 10.1185/03007998109114287. [DOI] [PubMed] [Google Scholar]
- Kendall M. D., al-Shawaf A. A. Innervation of the rat thymus gland. Brain Behav Immun. 1991 Mar;5(1):9–28. doi: 10.1016/0889-1591(91)90004-t. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D., Sprent J. Identity of cells that imprint H-2-restricted T-cell specificity in the thymus. Nature. 1986 Feb 20;319(6055):672–675. doi: 10.1038/319672a0. [DOI] [PubMed] [Google Scholar]
- McCall T., Vallance P. Nitric oxide takes centre-stage with newly defined roles. Trends Pharmacol Sci. 1992 Jan;13(1):1–6. doi: 10.1016/0165-6147(92)90002-n. [DOI] [PubMed] [Google Scholar]
- Mills C. D. Molecular basis of "suppressor" macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991 Apr 15;146(8):2719–2723. [PubMed] [Google Scholar]
- Mocchegiani E., Cacciatore L., Talarico M., Lingetti M., Fabris N. Recovery of low thymic hormone levels in cancer patients by lysine-arginine combination. Int J Immunopharmacol. 1990;12(4):365–371. doi: 10.1016/0192-0561(90)90017-h. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pelletier M., Tautu C., Landry D., Montplaisir S., Chartrand C., Perreault C. Characterization of human thymic dendritic cells in culture. Immunology. 1986 Jun;58(2):263–270. [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready A. R., Jenkinson E. J., Kingston R., Owen J. J. Successful transplantation across major histocompatibility barrier of deoxyguanosine-treated embryonic thymus expressing class II antigens. Nature. 1984 Jul 19;310(5974):231–233. doi: 10.1038/310231a0. [DOI] [PubMed] [Google Scholar]
- Sagar S. M. NADPH-diaphorase reactive neurons of the rabbit retina: differential sensitivity to excitotoxins and unusual morphologic features. J Comp Neurol. 1990 Oct 15;300(3):309–319. doi: 10.1002/cne.903000304. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Nitric oxide as a neuronal messenger. Trends Pharmacol Sci. 1991 Apr;12(4):125–128. doi: 10.1016/0165-6147(91)90526-x. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Nitric oxide and neurons. Curr Opin Neurobiol. 1992 Jun;2(3):323–327. doi: 10.1016/0959-4388(92)90123-3. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46(4):755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Von Boehmer H., Schubiger K. Thymocytes appear to ignore class I major histocompatibility complex antigens expressed on thymus epithelial cells. Eur J Immunol. 1984 Nov;14(11):1048–1052. doi: 10.1002/eji.1830141116. [DOI] [PubMed] [Google Scholar]
- Yui Y., Hattori R., Kosuga K., Eizawa H., Hiki K., Ohkawa S., Ohnishi K., Terao S., Kawai C. Calmodulin-independent nitric oxide synthase from rat polymorphonuclear neutrophils. J Biol Chem. 1991 Feb 25;266(6):3369–3371. [PubMed] [Google Scholar]