Abstract
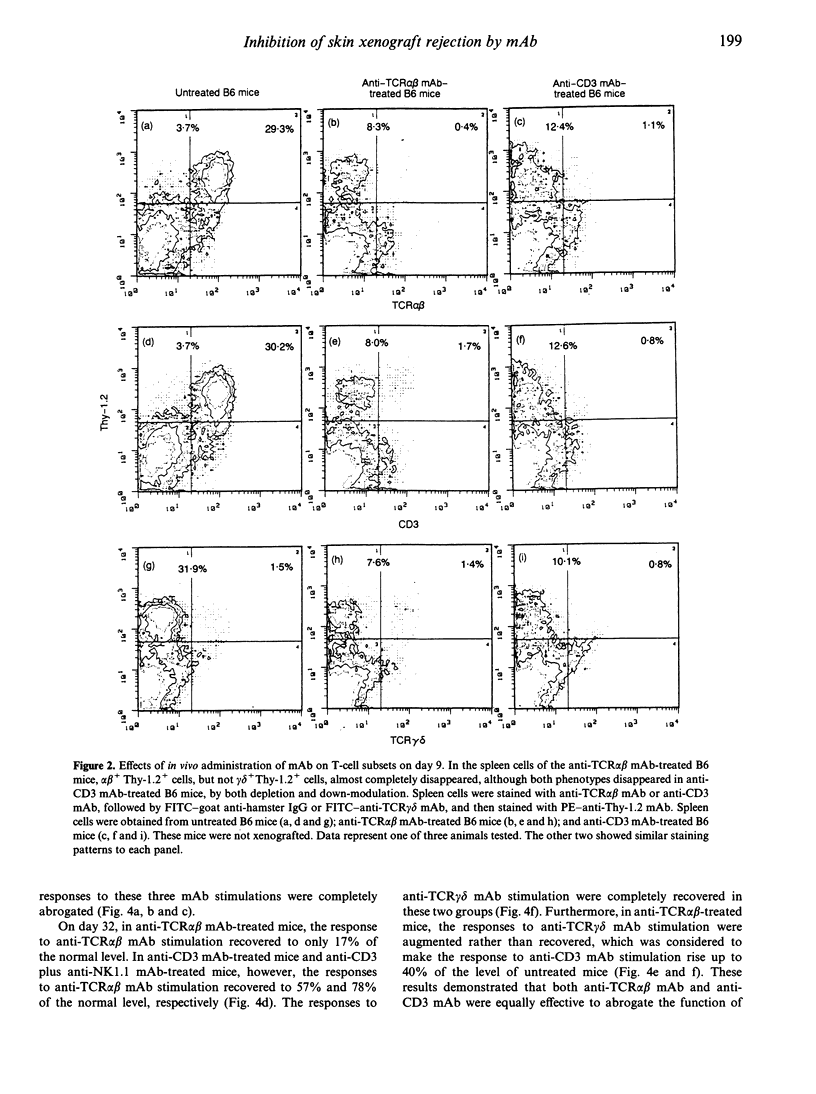
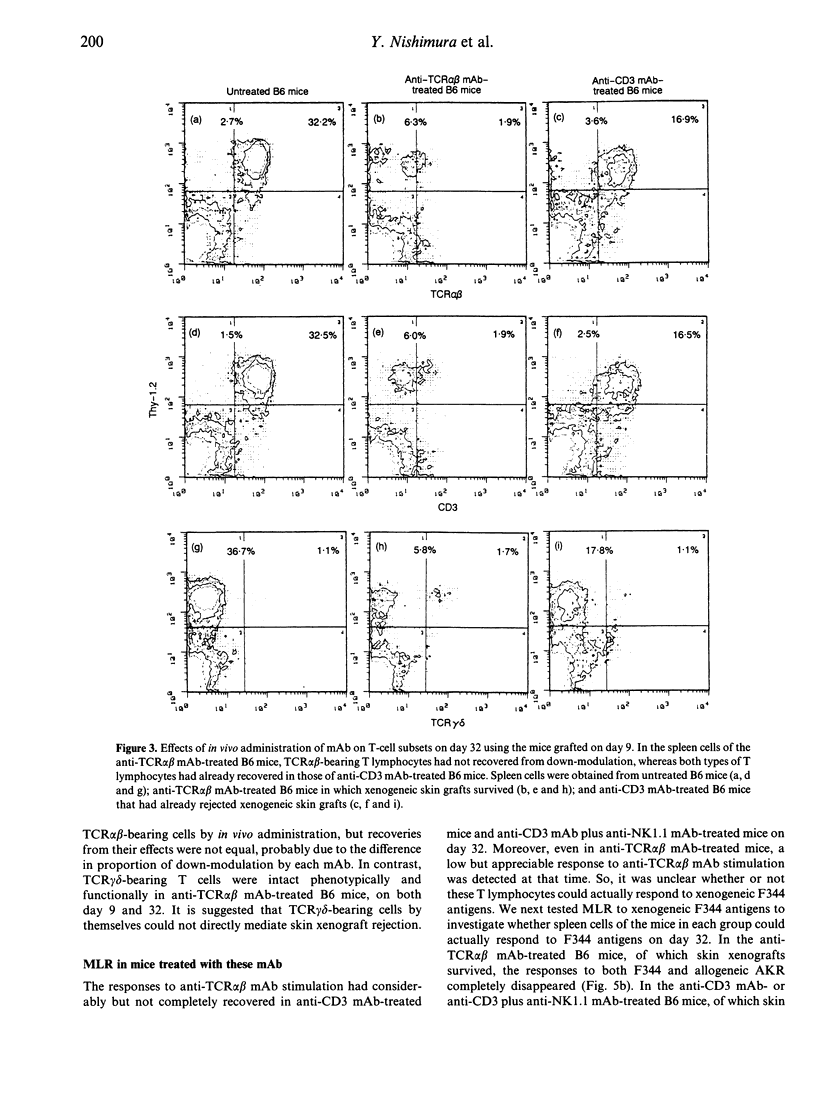
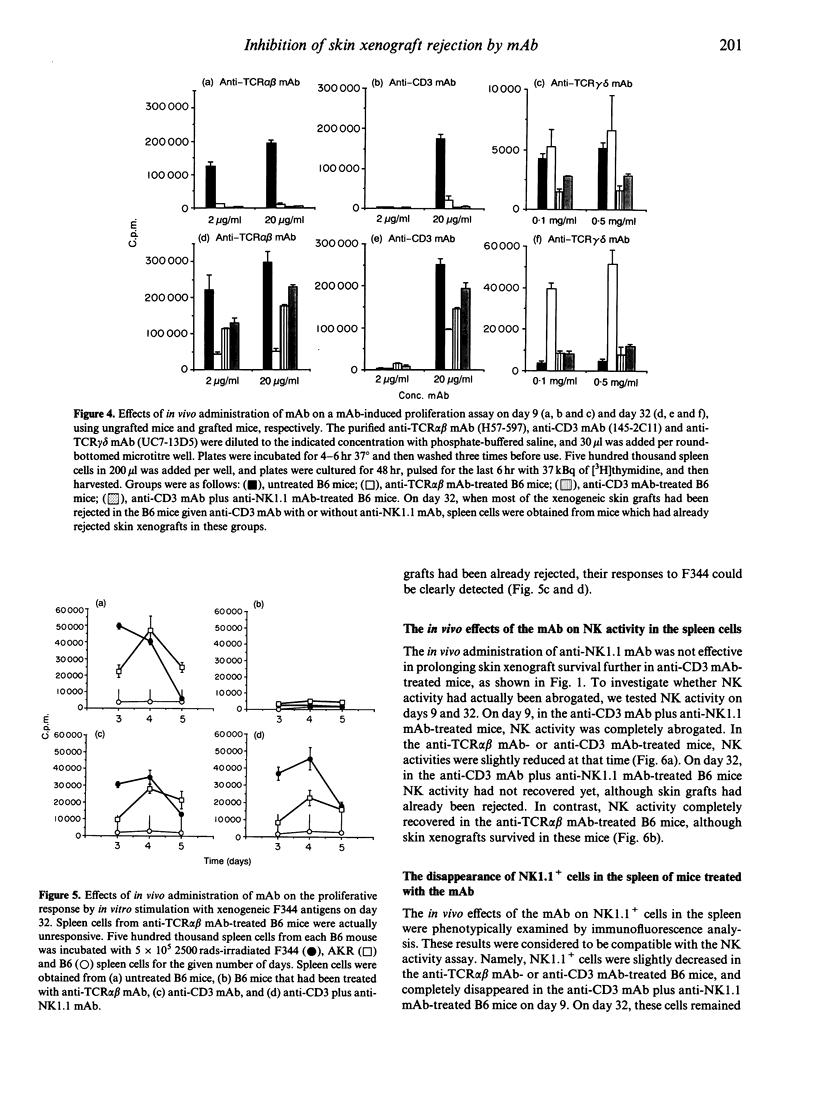
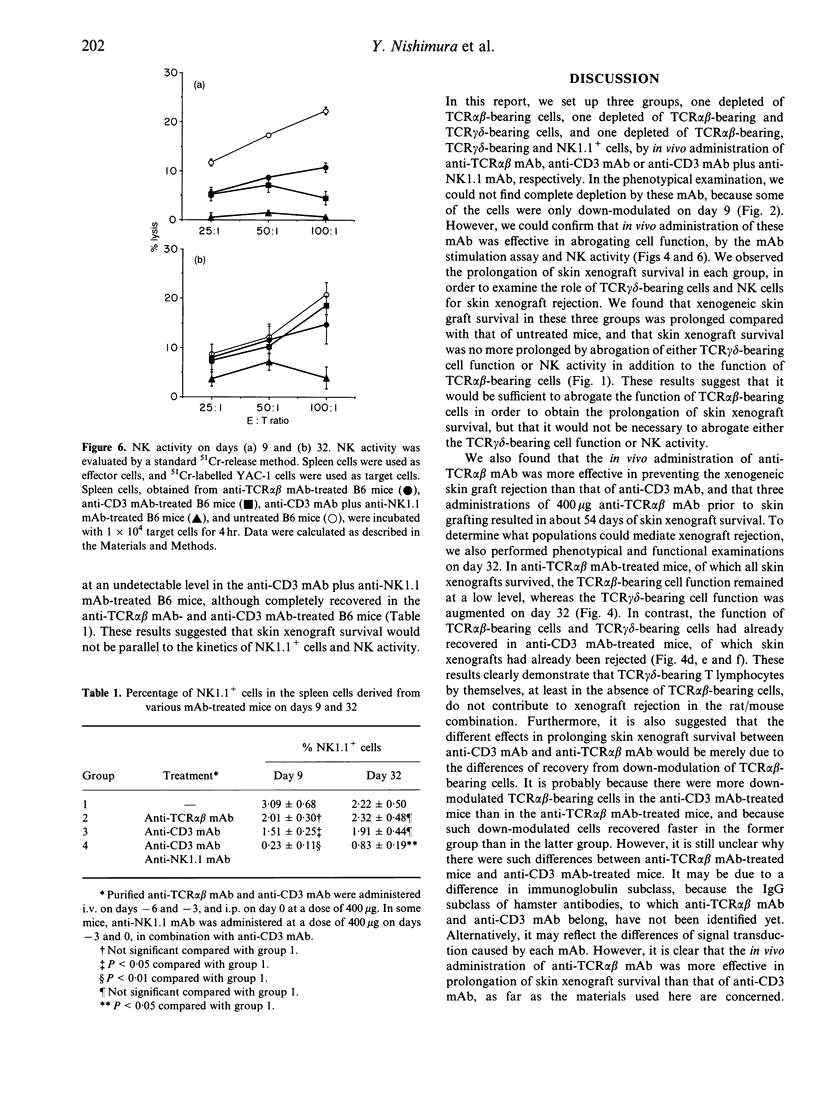
We compared the effects of in vivo administration of the anti-T-cell receptor (TCR) alpha beta monoclonal antibody (mAb) (H57-597) to those of the anti-CD3 mAb (145-2C11), with or without anti-NK1.1 mAb (PK136), on xenogeneic skin graft survival in mice. In anti-TCR alpha beta mAb-treated B6 mice, F344 rat skin grafts survived for about 54 days, whereas in anti-CD3 mAb-treated B6 mice with or without anti-NK1.1 mAb treatment grafts survived about 25 days. In anti-TCR alpha beta mAb-treated B6 mice, TCR alpha beta-bearing T-lymphocyte function was completely abrogated, although TCR gamma delta-bearing T-lymphocyte function was still intact on day 9. In the anti-CD3 mAb-treated mice, the functions of both types of T lymphocytes were completely abrogated. On day 32, when most of the skin xenografts had been rejected in the anti-CD3 mAb-treated mice, the functions of both T lymphocytes had recovered considerably, and could actually respond to F344 antigens. In contrast, the function of TCR alpha beta-bearing cells had only partially recovered in the anti-TCR alpha beta mAb-treated mice. Finally, natural killer (NK) activity in the anti-TCR alpha beta mAb-treated mice was intact on day 32, when rat skin grafts still survived. In contrast, NK activity in the anti-CD3 mAb plus anti-NK1.1 mAb-treated mice did not recover on day 32, when skin xenografts had already been rejected. These results suggest that TCR gamma delta-bearing T cells and NK cells by themselves, at least in the absence of TCR alpha beta-bearing T cells, do not mediate xenogeneic skin graft rejection in mouse/rat combinations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksentijevich I., Sachs D. H., Sykes M. Natural antibodies can inhibit bone marrow engraftment in the rat----mouse species combination. J Immunol. 1991 Dec 15;147(12):4140–4146. [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Dialynas D. P., Strominger J. L., Smith J. A., Owen F. L., Seidman J. G., Ip S., Rosen F., Krangel M. S. Identification of a putative second T-cell receptor. Nature. 1986 Jul 10;322(6075):145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- Carbone A., Harbeck R., Dallas A., Nemazee D., Finkel T., O'Brien R., Kubo R., Born W. Alpha beta T-lymphocyte depleted mice, a model for gamma delta T-lymphocyte functional studies. Immunol Rev. 1991 Apr;120:35–50. doi: 10.1111/j.1600-065x.1991.tb00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L., Ferran C., Legendre C., Kurrle R., Kreis H., Bach J. F. Immunological follow-up of renal allograft recipients treated with the BMA 031 (anti-TCR) monoclonal antibody. Transplant Proc. 1990 Aug;22(4):1787–1788. [PubMed] [Google Scholar]
- Ciccone E., Viale O., Bottino C., Pende D., Migone N., Casorati G., Tambussi G., Moretta A., Moretta L. Antigen recognition by human T cell receptor gamma-positive lymphocytes. Specific lysis of allogeneic cells after activation in mixed lymphocyte culture. J Exp Med. 1988 Apr 1;167(4):1517–1522. doi: 10.1084/jem.167.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cron R. Q., Gajewski T. F., Sharrow S. O., Fitch F. W., Matis L. A., Bluestone J. A. Phenotypic and functional analysis of murine CD3+,CD4-,CD8- TCR-gamma delta-expressing peripheral T cells. J Immunol. 1989 Jun 1;142(11):3754–3762. [PubMed] [Google Scholar]
- Dendorfer U., Hillebrand G., Kasper C., Smely S., Weschka M., Hammer C., Racenberg J., Gurland H. J., Land W. Effective prevention of interstitial rejection crises in immunological high risk patients following renal transplantation: use of high doses of the new monoclonal antibody BMA 031. Transplant Proc. 1990 Aug;22(4):1789–1790. [PubMed] [Google Scholar]
- Eto M., Yoshikai Y., Nishimura Y., Hiromatsu K., Maeda T., Nomoto K., Kong Y. Y., Kubo R. T., Kumazawa J., Nomoto K. Inhibition of allograft rejection by anti-T-cell receptor-alpha beta monoclonal antibodies preserving resistance to bacterial infection. Immunology. 1994 Feb;81(2):198–204. [PMC free article] [PubMed] [Google Scholar]
- Ferran C., Sheehan K., Dy M., Schreiber R., Merite S., Landais P., Noel L. H., Grau G., Bluestone J., Bach J. F. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol. 1990 Mar;20(3):509–515. doi: 10.1002/eji.1830200308. [DOI] [PubMed] [Google Scholar]
- First M. R. Transplantation in the nineties. Transplantation. 1992 Jan;53(1):1–11. doi: 10.1097/00007890-199201000-00001. [DOI] [PubMed] [Google Scholar]
- Hiromatsu K., Yoshikai Y., Matsuzaki G., Ohga S., Muramori K., Matsumoto K., Bluestone J. A., Nomoto K. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992 Jan 1;175(1):49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R., Archibald J., Gress R. E. Differential T cell hyporesponsiveness induced by in vivo administration of intact or F(ab')2 fragments of anti-CD3 monoclonal antibody. F(ab')2 fragments induce a selective T helper dysfunction. J Immunol. 1991 Oct 1;147(7):2088–2093. [PubMed] [Google Scholar]
- Hirsch R., Bluestone J. A., DeNenno L., Gress R. E. Anti-CD3 F(ab')2 fragments are immunosuppressive in vivo without evoking either the strong humoral response or morbidity associated with whole mAb. Transplantation. 1990 Jun;49(6):1117–1123. doi: 10.1097/00007890-199006000-00018. [DOI] [PubMed] [Google Scholar]
- Hirsch R., Eckhaus M., Auchincloss H., Jr, Sachs D. H., Bluestone J. A. Effects of in vivo administration of anti-T3 monoclonal antibody on T cell function in mice. I. Immunosuppression of transplantation responses. J Immunol. 1988 Jun 1;140(11):3766–3772. [PubMed] [Google Scholar]
- Hirsch R., Gress R. E., Pluznik D. H., Eckhaus M., Bluestone J. A. Effects of in vivo administration of anti-CD3 monoclonal antibody on T cell function in mice. II. In vivo activation of T cells. J Immunol. 1989 Feb 1;142(3):737–743. [PubMed] [Google Scholar]
- Inoue T., Yoshikai Y., Matsuzaki G., Nomoto K. Early appearing gamma/delta-bearing T cells during infection with Calmétte Guérin bacillus. J Immunol. 1991 Apr 15;146(8):2754–2762. [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Levitsky H. I., Golumbek P. T., Pardoll D. M. The fate of CD4-8- T cell receptor-alpha beta+ thymocytes. J Immunol. 1991 Feb 15;146(4):1113–1117. [PubMed] [Google Scholar]
- Lim S. M., Wee A., Chong S. M., White D. J. An analysis of concordant xenograft rejection in the nude rat model. Transplant Proc. 1990 Oct;22(5):2095–2096. [PubMed] [Google Scholar]
- Lucas P. J., Shearer G. M., Neudorf S., Gress R. E. The human antimurine xenogeneic cytotoxic response. I. Dependence on responder antigen-presenting cells. J Immunol. 1990 Jun 15;144(12):4548–4554. [PubMed] [Google Scholar]
- Matis L. A., Cron R., Bluestone J. A. Major histocompatibility complex-linked specificity of gamma delta receptor-bearing T lymphocytes. Nature. 1987 Nov 19;330(6145):262–264. doi: 10.1038/330262a0. [DOI] [PubMed] [Google Scholar]
- Mayumi H., Nomoto K., Good R. A. A surgical technique for experimental free skin grafting in mice. Jpn J Surg. 1988 Sep;18(5):548–557. doi: 10.1007/BF02471489. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Moses R. D., Pierson R. N., 3rd, Winn H. J., Auchincloss H., Jr Xenogeneic proliferation and lymphokine production are dependent on CD4+ helper T cells and self antigen-presenting cells in the mouse. J Exp Med. 1990 Aug 1;172(2):567–575. doi: 10.1084/jem.172.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga S., Yoshikai Y., Takeda Y., Hiromatsu K., Nomoto K. Sequential appearance of gamma/delta- and alpha/beta-bearing T cells in the peritoneal cavity during an i.p. infection with Listeria monocytogenes. Eur J Immunol. 1990 Mar;20(3):533–538. doi: 10.1002/eji.1830200311. [DOI] [PubMed] [Google Scholar]
- Pierson R. N., 3rd, Winn H. J., Russell P. S., Auchincloss H., Jr Xenogeneic skin graft rejection is especially dependent on CD4+ T cells. J Exp Med. 1989 Sep 1;170(3):991–996. doi: 10.1084/jem.170.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabi Y., Aksentijevich I., Sundt T. M., 3rd, Sachs D. H., Sykes M. Specific tolerance induction across a xenogeneic barrier: production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. J Exp Med. 1990 Jul 1;172(1):195–202. doi: 10.1084/jem.172.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smely S., Weschka M., Hillebrand G., Dendorfer U., Krombach F., Kurrle R., Land W., Hammer C. Prophylactic use of the new monoclonal antibody BMA 031 in clinical kidney transplantation. Transplant Proc. 1990 Aug;22(4):1785–1786. [PubMed] [Google Scholar]
- Sykes M. Unusual T cell populations in adult murine bone marrow. Prevalence of CD3+CD4-CD8- and alpha beta TCR+NK1.1+ cells. J Immunol. 1990 Nov 15;145(10):3209–3215. [PubMed] [Google Scholar]
- Thistlethwaite J. R., Jr, Cosimi A. B., Delmonico F. L., Rubin R. H., Talkoff-Rubin N., Nelson P. W., Fang L., Russell P. S. Evolving use of OKT3 monoclonal antibody for treatment of renal allograft rejection. Transplantation. 1984 Dec;38(6):695–701. doi: 10.1097/00007890-198412000-00029. [DOI] [PubMed] [Google Scholar]
- Thomas F. T., Marchman W., Carobbi A., Araneda D., Pryor W., Thomas J. Immunobiology of the xenograft response: xenograft rejection in immunodeficient animals. Transplant Proc. 1991 Feb;23(1 Pt 1):208–209. [PubMed] [Google Scholar]
- Vigeral P., Chkoff N., Chatenoud L., Campos H., Lacombe M., Droz D., Goldstein G., Bach J. F., Kreis H. Prophylactic use of OKT3 monoclonal antibody in cadaver kidney recipients. Utilization of OKT3 as the sole immunosuppressive agent. Transplantation. 1986 Jun;41(6):730–733. doi: 10.1097/00007890-198606000-00013. [DOI] [PubMed] [Google Scholar]
- Yankelevich B., Knobloch C., Nowicki M., Dennert G. A novel cell type responsible for marrow graft rejection in mice. T cells with NK phenotype cause acute rejection of marrow grafts. J Immunol. 1989 May 15;142(10):3423–3430. [PubMed] [Google Scholar]


