Abstract
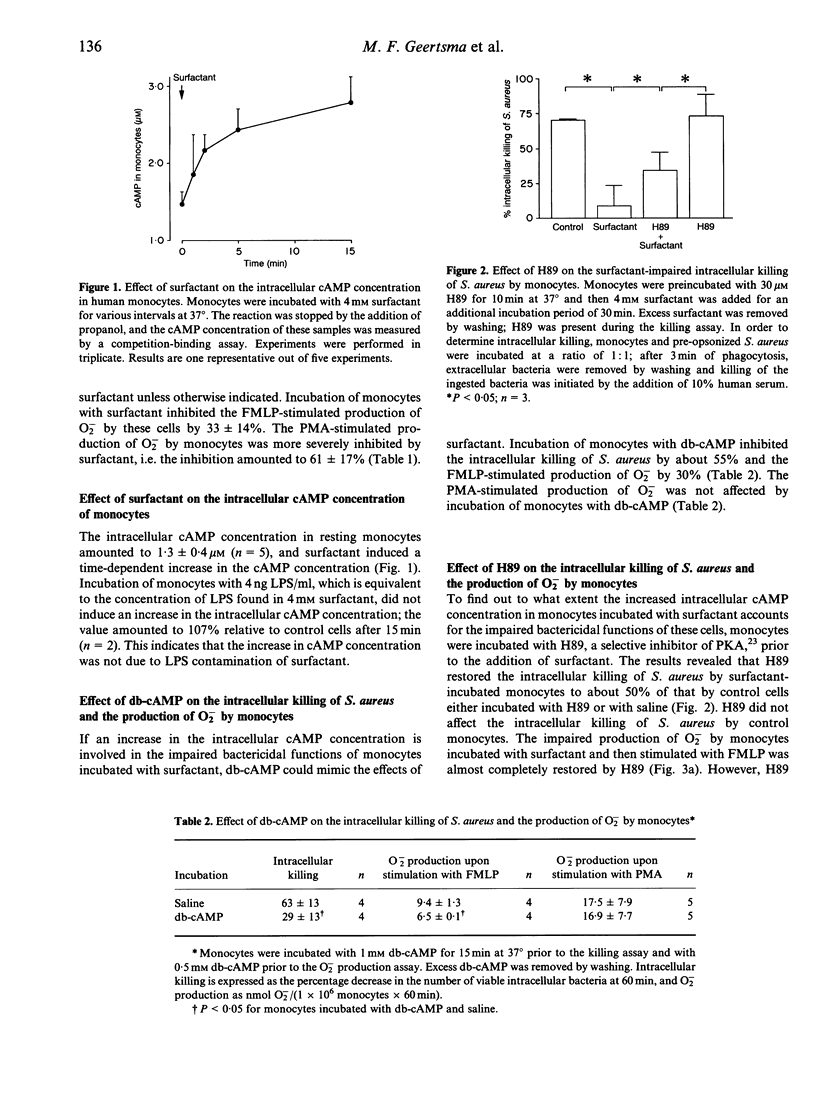
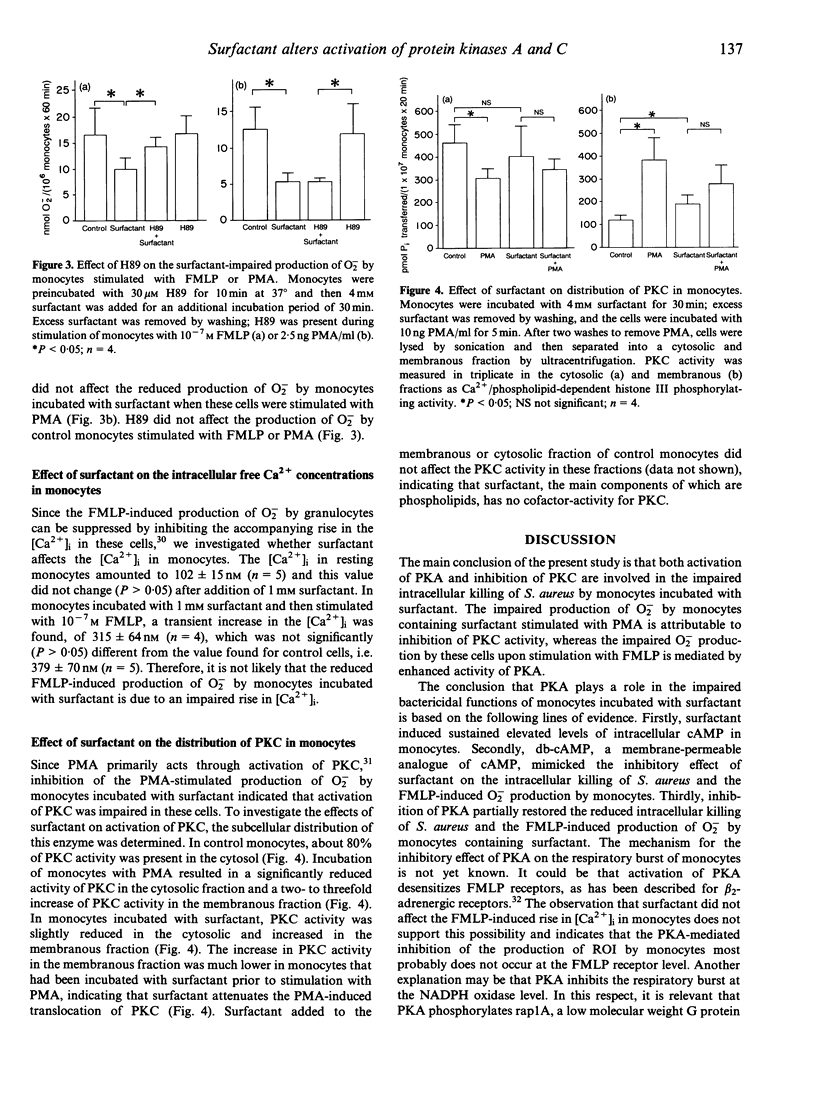
Pulmonary surfactant, the main function of which is to reduce surface tension in the alveoli, is also known to affect the functions of monocytes. Two protein kinases play a role in the regulation of the bactericidal functions of phagocytes, i.e. cAMP-dependent protein kinase A (PKA), which is involved in inhibition, and Ca2+/phospholipid-dependent PKC, which is involved in stimulation of these functions. In the present study we investigated whether altered activation of PKA and/or PKC plays a role in the surfactant-induced inhibition of both the intracellular killing of Staphylococcus aureus and the production of reactive oxygen intermediates (ROI) by monocytes. The significance of increased activation of PKA was demonstrated by the following findings. Firstly, surfactant induced a sustained increase in the intracellular cAMP concentration in monocytes. Secondly, dibutyryl-cAMP (db-cAMP), a membrane-permeable cAMP analogue, mimicked the inhibitory effects of surfactant on both the killing capacity and the production of ROI by monocytes. Thirdly, an inhibitor of PKA partially restored the impaired bactericidal functions of monocytes incubated with surfactant. The involvement of decreased activation of PKC in the impaired bactericidal functions of monocytes incubated with surfactant was evident from two findings. Firstly, surfactant attenuated the phorbol myristate acetate (PMA)-mediated translocation of PKC. Secondly, surfactant inhibited the production of O2- by monocytes upon stimulation with PMA. Therefore, the mechanism involved in the surfactant-induced inhibition of the bactericidal functions of monocytes comprises both activation of an inhibitory pathway, which includes cAMP and PKA, and inactivation of a stimulatory pathway, in which PKC is involved.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Knippenberg R. W., Bjornson H. S. Bactericidal defect of neutrophils in a guinea pig model of thermal injury is related to elevation of intracellular cyclic-3',5'-adenosine monophosphate. J Immunol. 1989 Oct 15;143(8):2609–2616. [PubMed] [Google Scholar]
- Brieland J. K., Balazovich K., Fantone J. C. The effect of acute inflammatory lung injury on the respiratory burst and protein kinase C activity of rat pulmonary alveolar macrophages. Am Rev Respir Dis. 1989 Feb;139(2):378–381. doi: 10.1164/ajrccm/139.2.378. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Channon J. Y., Leslie C. C., Johnston R. B., Jr Zymosan-stimulated production of phosphatidic acid by macrophages: relationship to release of superoxide anion and inhibition by agents that increase intracellular cyclic AMP. J Leukoc Biol. 1987 May;41(5):450–453. doi: 10.1002/jlb.41.5.450. [DOI] [PubMed] [Google Scholar]
- Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990 Mar 25;265(9):5267–5272. [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haagsman H. P., van Golde L. M. Synthesis and assembly of lung surfactant. Annu Rev Physiol. 1991;53:441–464. doi: 10.1146/annurev.ph.53.030191.002301. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Schmeling D., Peterson P. K. Phagocytosis, bacterial killing, and metabolism by purified human lung phagocytes. J Infect Dis. 1981 Jul;144(1):61–71. doi: 10.1093/infdis/144.1.61. [DOI] [PubMed] [Google Scholar]
- Kessels G. C., Krause K. H., Verhoeven A. J. Protein kinase C activity is not involved in N-formylmethionyl-leucyl-phenylalanine-induced phospholipase D activation in human neutrophils, but is essential for concomitant NADPH oxidase activation: studies with a staurosporine analogue with improved selectivity for protein kinase C. Biochem J. 1993 Jun 15;292(Pt 3):781–785. doi: 10.1042/bj2920781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I. M., Verhoeven A. J., van der Bend R. L., Weening R. S., Roos D. Purified protein kinase C phosphorylates a 47-kDa protein in control neutrophil cytoplasts but not in neutrophil cytoplasts from patients with the autosomal form of chronic granulomatous disease. J Biol Chem. 1988 Feb 15;263(5):2352–2357. [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie D. B., Jackett P. S., Ratcliffe N. A. Mycobacterium microti may protect itself from intracellular destruction by releasing cyclic AMP into phagosomes. Nature. 1975 Apr 17;254(5501):600–602. doi: 10.1038/254600a0. [DOI] [PubMed] [Google Scholar]
- Majumdar S., Rossi M. W., Fujiki T., Phillips W. A., Disa S., Queen C. F., Johnston R. B., Jr, Rosen O. M., Corkey B. E., Korchak H. M. Protein kinase C isotypes and signaling in neutrophils. Differential substrate specificities of a translocatable calcium- and phospholipid-dependent beta-protein kinase C and a phospholipid-dependent protein kinase which is inhibited by long chain fatty acyl coenzyme A. J Biol Chem. 1991 May 15;266(14):9285–9294. [PubMed] [Google Scholar]
- Mueller H., Montoya B., Sklar L. A. Reversal of inhibitory pathways in neutrophils by protein kinase antagonists: a rational approach to the restoration of depressed cell function? J Leukoc Biol. 1992 Oct;52(4):400–406. doi: 10.1002/jlb.52.4.400. [DOI] [PubMed] [Google Scholar]
- Myers M. A., McPhail L. C., Snyderman R. Redistribution of protein kinase C activity in human monocytes: correlation with activation of the respiratory burst. J Immunol. 1985 Nov;135(5):3411–3416. [PubMed] [Google Scholar]
- Nauseef W. M., Volpp B. D., McCormick S., Leidal K. G., Clark R. A. Assembly of the neutrophil respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J Biol Chem. 1991 Mar 25;266(9):5911–5917. [PubMed] [Google Scholar]
- Nibbering P. H., Zomerdijk T. P., Corsèl-Van Tilburg A. J., Van Furth R. Mean cell volume of human blood leucocytes and resident and activated murine macrophages. J Immunol Methods. 1990 May 8;129(1):143–145. doi: 10.1016/0022-1759(90)90432-u. [DOI] [PubMed] [Google Scholar]
- Nichols B. A. Normal rabbit alveolar macrophages. I. The phagocytosis of tubular myelin. J Exp Med. 1976 Oct 1;144(4):906–919. doi: 10.1084/jem.144.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dorisio M. S., Vandenbark G. R., LoBuglio A. F. Human monocyte killing of Staphylococcus aureus: modulation by agonists of cyclic adenosine 3',5'-monophosphate and cyclic guanosine 3',5'-monophosphate. Infect Immun. 1979 Nov;26(2):604–610. doi: 10.1128/iai.26.2.604-610.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-Golden M., McNish R. W., Sporn P. H., Balazovich K. Basal activation of protein kinase C in rat alveolar macrophages: implications for arachidonate metabolism. Am J Physiol. 1991 Dec;261(6 Pt 1):L462–L471. doi: 10.1152/ajplung.1991.261.6.L462. [DOI] [PubMed] [Google Scholar]
- Quilliam L. A., Mueller H., Bohl B. P., Prossnitz V., Sklar L. A., Der C. J., Bokoch G. M. Rap1A is a substrate for cyclic AMP-dependent protein kinase in human neutrophils. J Immunol. 1991 Sep 1;147(5):1628–1635. [PubMed] [Google Scholar]
- Sedgwick J. B., Berube M. L., Zurier R. B. Stimulus-dependent inhibition of superoxide generation by prostaglandins. Clin Immunol Immunopathol. 1985 Feb;34(2):205–215. doi: 10.1016/0090-1229(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Seeber F., Brattig N., Soboslay P. T., Pogonka T., Lörz A., Strote G., Beck E., Titanji V. P., Lucius R. Characterization of a recombinant T cell and B cell reactive polypeptide of Onchocerca volvulus. J Immunol. 1993 Apr 1;150(7):2931–2944. [PubMed] [Google Scholar]
- Snoek G. T., Boonstra J., Ponec M., de Laat S. W. Phorbol ester binding and protein kinase C activity in normal and transformed human keratinocytes. Exp Cell Res. 1987 Sep;172(1):146–157. doi: 10.1016/0014-4827(87)90101-7. [DOI] [PubMed] [Google Scholar]
- Wilson E., Olcott M. C., Bell R. M., Merrill A. H., Jr, Lambeth J. D. Inhibition of the oxidative burst in human neutrophils by sphingoid long-chain bases. Role of protein kinase C in activation of the burst. J Biol Chem. 1986 Sep 25;261(27):12616–12623. [PubMed] [Google Scholar]
- Wolfson M., McPhail L. C., Nasrallah V. N., Snyderman R. Phorbol myristate acetate mediates redistribution of protein kinase C in human neutrophils: potential role in the activation of the respiratory burst enzyme. J Immunol. 1985 Sep;135(3):2057–2062. [PubMed] [Google Scholar]
- Zeligs B. J., Nerurkar L. S., Bellanti J. A., Zeligs J. D. Maturation of the rabbit alveolar macrophage during animal development. I. Perinatal influx into alveoli and ultrastructural differentiation. Pediatr Res. 1977 Mar;11(3 Pt 1):197–208. doi: 10.1203/00006450-197703000-00011. [DOI] [PubMed] [Google Scholar]
- Zheng L., Nibbering P. H., van Furth R. Stimulation of the intracellular killing of Staphylococcus aureus by human monocytes mediated by Fc gamma receptors I and II. Eur J Immunol. 1993 Nov;23(11):2826–2833. doi: 10.1002/eji.1830231116. [DOI] [PubMed] [Google Scholar]
- Zomerdijk T. P., Nibbering P. H., Bezemer A. C., Löwik C. W., van Furth R. A new competition binding assay for determination of the cAMP content of human leukocytes. Biochem Biophys Res Commun. 1991 Aug 15;178(3):980–984. doi: 10.1016/0006-291x(91)90988-j. [DOI] [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]


