Abstract
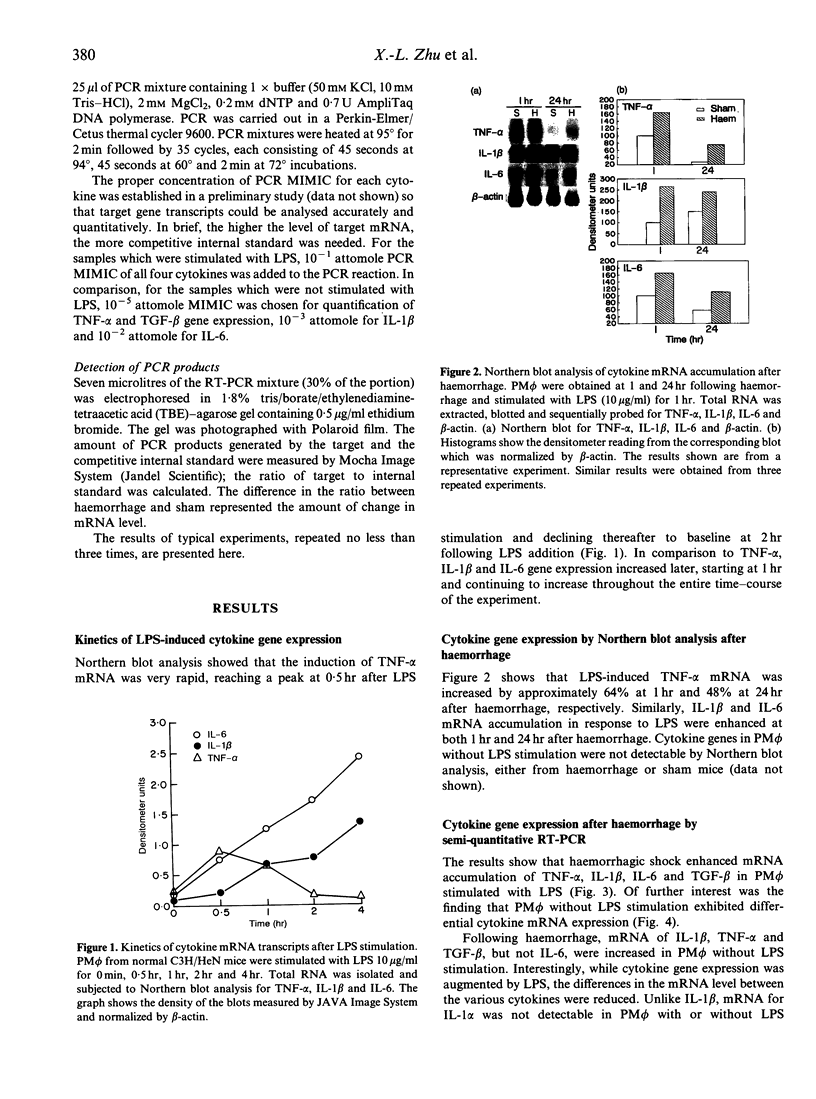
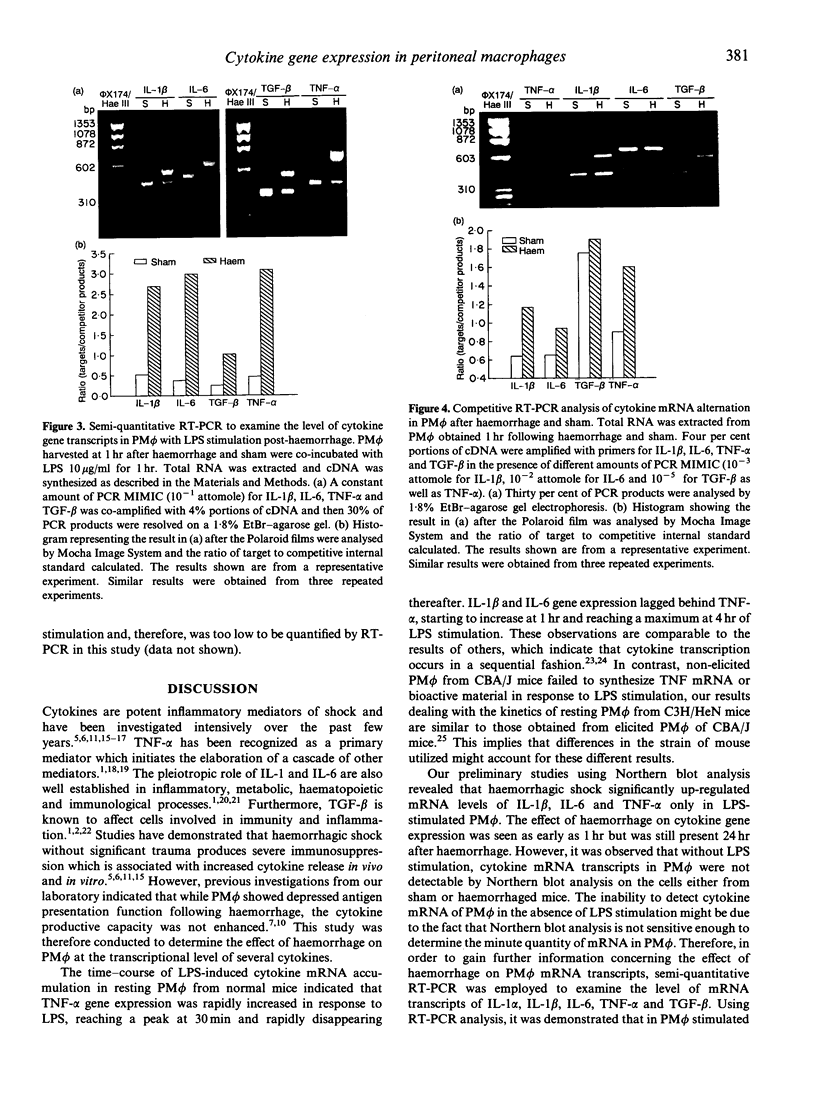
Tumour necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), IL-1 and transforming growth factor-beta (TGF-beta) have been recognized as important mediators of pathophysiological and immunological events associated with shock. Previous studies have indicated that although peritoneal macrophage (PM phi) antigen presentation was depressed following haemorrhage, the cytokine release capacity in response to lipopolysaccharide (LPS) was not affected in vitro. To determine the effect of haemorrhagic shock on PM phi cytokine mRNA transcription, C3H/HeN male mice were bled to and maintained at a mean arterial blood pressure of 35 mmHg for 60 min, and then adequately resuscitated. PM phi were isolated at 1 or 24 hr after haemorrhage and were incubated without or with 10 micrograms LPS/ml for 1 hr. Total RNA was then extracted followed by Northern blot analysis, as well as semi-quantitative reverse transcription and polymerase chain reaction (RT-PCR). The results of Northern blot analysis indicated that haemorrhage markedly increased LPS-induced IL-1 beta, IL-6, and TNF-alpha mRNA accumulation in PM phi at both 1 and 24 hr after haemorrhage and resuscitation. Furthermore, competitive RT-PCR demonstrated that mRNA of IL-1 beta, IL-6, TNF-alpha, as well as TGF-beta, was increased in PM phi obtained 1 hr after haemorrhage either with or without LPS stimulation. The data from Northern blot analysis and semi-quantitative RT-PCR also revealed that LPS enhanced the effect of haemorrhage on PM phi cytokine gene expression. Thus, following haemorrhage, PM phi showed elevated cytokine mRNA accumulation which was not followed by an increased ability to release cytokines in response to LPS in vitro. These results, therefore, suggest that different mechanisms regulate gene expression and subsequent cytokine secretion by PM phi following haemorrhage.
Full text
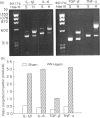
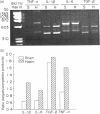
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E., Richmond N. J., Chang Y. H. Effects of hemorrhage on interleukin-1 production. Circ Shock. 1988 May;25(1):33–40. [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A., Meldrum D. R., Perrin M. M., Chaudry I. H. The release of transforming growth factor-beta following haemorrhage: its role as a mediator of host immunosuppression. Immunology. 1993 Jul;79(3):479–484. [PMC free article] [PubMed] [Google Scholar]
- Ayala A., Perrin M. M., Chaudry I. H. Defective macrophage antigen presentation following haemorrhage is associated with the loss of MHC class II (Ia) antigens. Immunology. 1990 May;70(1):33–39. [PMC free article] [PubMed] [Google Scholar]
- Ayala A., Perrin M. M., Ertel W., Chaudry I. H. Differential effects of hemorrhage on Kupffer cells: decreased antigen presentation despite increased inflammatory cytokine (IL-1, IL-6 and TNF) release. Cytokine. 1992 Jan;4(1):66–75. doi: 10.1016/1043-4666(92)90039-t. [DOI] [PubMed] [Google Scholar]
- Ayala A., Perrin M. M., Meldrum D. R., Ertel W., Chaudry I. H. Hemorrhage induces an increase in serum TNF which is not associated with elevated levels of endotoxin. Cytokine. 1990 May;2(3):170–174. doi: 10.1016/1043-4666(90)90012-i. [DOI] [PubMed] [Google Scholar]
- Ayala A., Perrin M. M., Wang P., Ertel W., Chaudry I. H. Hemorrhage induces enhanced Kupffer cell cytotoxicity while decreasing peritoneal or splenic macrophage capacity. Involvement of cell-associated tumor necrosis factor and reactive nitrogen. J Immunol. 1991 Dec 15;147(12):4147–4154. [PubMed] [Google Scholar]
- Ayala A., Wang P., Ba Z. F., Perrin M. M., Ertel W., Chaudry I. H. Differential alterations in plasma IL-6 and TNF levels after trauma and hemorrhage. Am J Physiol. 1991 Jan;260(1 Pt 2):R167–R171. doi: 10.1152/ajpregu.1991.260.1.R167. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Damas P., Ledoux D., Nys M., Vrindts Y., De Groote D., Franchimont P., Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992 Apr;215(4):356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Ertel W., Morrison M. H., Ayala A., Chaudry I. H. Insights into the mechanisms of defective antigen presentation after hemorrhage. Surgery. 1991 Aug;110(2):440–447. [PubMed] [Google Scholar]
- Ertel W., Morrison M. H., Ayala A., Perrin M. M., Chaudry I. H. Blockade of prostaglandin production increases cachectin synthesis and prevents depression of macrophage functions after hemorrhagic shock. Ann Surg. 1991 Mar;213(3):265–271. doi: 10.1097/00000658-199103000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton M. J. Review: transcriptional and post-transcriptional regulation of interleukin 1 gene expression. Int J Immunopharmacol. 1992 Apr;14(3):401–411. doi: 10.1016/0192-0561(92)90170-p. [DOI] [PubMed] [Google Scholar]
- Fong Y., Tracey K. J., Moldawer L. L., Hesse D. G., Manogue K. B., Kenney J. S., Lee A. T., Kuo G. C., Allison A. C., Lowry S. F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989 Nov 1;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas E., Sargent T. D., Dawid I. B. Epidermal keratin gene expressed in embryos of Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5413–5417. doi: 10.1073/pnas.82.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. A., Dorf M. E. Differential regulation of interleukin-6, macrophage inflammatory protein-1, and JE/MCP-1 cytokine expression in macrophage cell lines. Cell Immunol. 1991 Jun;135(1):245–258. doi: 10.1016/0008-8749(91)90269-h. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Remick D. G., Strieter R. M., Lynch J. P., 3rd, Nguyen D., Eskandari M., Kunkel S. L. In vivo dynamics of murine tumor necrosis factor-alpha gene expression. Kinetics of dexamethasone-induced suppression. Lab Invest. 1989 Jun;60(6):766–771. [PubMed] [Google Scholar]
- Scales W. E., Chensue S. W., Otterness I., Kunkel S. L. Regulation of monokine gene expression: prostaglandin E2 suppresses tumor necrosis factor but not interleukin-1 alpha or beta-mRNA and cell-associated bioactivity. J Leukoc Biol. 1989 May;45(5):416–421. [PubMed] [Google Scholar]
- Stephan R. N., Kupper T. S., Geha A. S., Baue A. E., Chaudry I. H. Hemorrhage without tissue trauma produces immunosuppression and enhances susceptibility to sepsis. Arch Surg. 1987 Jan;122(1):62–68. doi: 10.1001/archsurg.1987.01400130068010. [DOI] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Mergenhagen S. E. Inflammatory and immunomodulatory roles of TGF-beta. Immunol Today. 1989 Aug;10(8):258–261. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]