Abstract
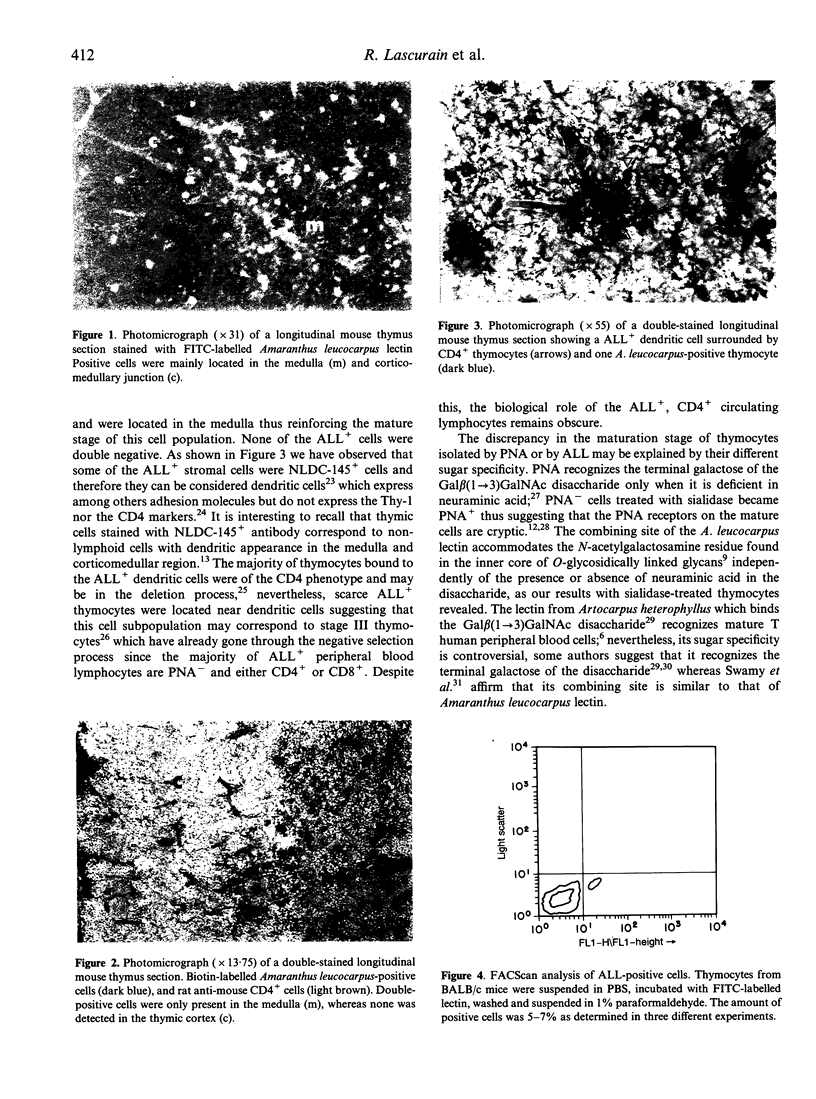
We have used the Gal beta(1-->3)GalNAc-specific Amaranthus leucocarpus lectin to isolate a thymus cell subpopulation which is different from that sorted with Arachis hypogaea lectin. The cells recognized by A. leucocarpus lectin were predominantly CD4+, whereas a minor proportion of CD8+ cells (approximately 11%) were also identified. The A. leucocarpus-positive cells were located in the thymus medulla and the cortico-medullary junction. The cortex was negative for A. leucocarpus cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Ardavin C., Shortman K. Cell surface marker analysis of mouse thymic dendritic cells. Eur J Immunol. 1992 Mar;22(3):859–862. doi: 10.1002/eji.1830220334. [DOI] [PubMed] [Google Scholar]
- Fowlkes B. J., Schwartz R. H., Pardoll D. M. Deletion of self-reactive thymocytes occurs at a CD4+8+ precursor stage. Nature. 1988 Aug 18;334(6183):620–623. doi: 10.1038/334620a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara K., Collet-Cassart D., Kobayashi K., Vaerman J. P. Jacalin: isolation, characterization, and influence of various factors on its interaction with human IgA1, as assessed by precipitation and latex agglutination. Mol Immunol. 1988 Jan;25(1):69–83. doi: 10.1016/0161-5890(88)90092-2. [DOI] [PubMed] [Google Scholar]
- Kamperdijk E. W., Kapsenberg M. L., van den Berg M., Hoefsmit E. C. Characterization of dendritic cells, isolated from normal and stimulated lymph nodes of the rat. Cell Tissue Res. 1985;242(3):469–474. doi: 10.1007/BF00225411. [DOI] [PubMed] [Google Scholar]
- Kraal G., Breel M., Janse M., Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986 Apr 1;163(4):981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- MacDonald H. R., Hengartner H., Pedrazzini T. Intrathymic deletion of self-reactive cells prevented by neonatal anti-CD4 antibody treatment. Nature. 1988 Sep 8;335(6186):174–176. doi: 10.1038/335174a0. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Lotan R., Ravid A., Sharon N. Peanut agglutinin, a new mitogen that binds to galactosyl sites exposed after neuraminidase treatment. J Immunol. 1975 Nov;115(5):1243–1248. [PubMed] [Google Scholar]
- Peacock J. S., Colsky A. S., Pinto V. B. Lectins and antibodies as tools for studying cellular interactions. J Immunol Methods. 1990 Feb 9;126(2):147–157. doi: 10.1016/0022-1759(90)90145-l. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Loures M. A., Villalta F., Andrade A. F. Lectin receptors as markers for Trypanosoma cruzi. Developmental stages and a study of the interaction of wheat germ agglutinin with sialic acid residues on epimastigote cells. J Exp Med. 1980 Nov 1;152(5):1375–1392. doi: 10.1084/jem.152.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau N., Aucouturier P., Brugier J. C., Preud'homme J. L. Jacalin: a lectin mitogenic for human CD4 T lymphocytes. Clin Exp Immunol. 1990 Jun;80(3):420–425. doi: 10.1111/j.1365-2249.1990.tb03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presant C. A., Kornfeld S. Characterization of the cell surface receptor for the Agaricus bisporus hemagglutinin. J Biol Chem. 1972 Nov 10;247(21):6937–6945. [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Roque-Barreira M. C., Campos-Neto A. Jacalin: an IgA-binding lectin. J Immunol. 1985 Mar;134(3):1740–1743. [PubMed] [Google Scholar]
- Scollay R., Bartlett P., Shortman K. T cell development in the adult murine thymus: changes in the expression of the surface antigens Ly2, L3T4 and B2A2 during development from early precursor cells to emigrants. Immunol Rev. 1984 Dec;82:79–103. doi: 10.1111/j.1600-065x.1984.tb01118.x. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Swamy M. J., Gupta D., Mahanta S. K., Surolia A. Further characterization of the saccharide specificity of peanut (Arachis hypogaea) agglutinin. Carbohydr Res. 1991 Jun 25;213:59–67. doi: 10.1016/s0008-6215(00)90598-6. [DOI] [PubMed] [Google Scholar]
- The T. H., Feltkamp T. E. Conjugation of fluorescein isothiocyanate to antibodies. I. Experiments on the conditions of conjugation. Immunology. 1970 Jun;18(6):865–873. [PMC free article] [PubMed] [Google Scholar]
- Zenteno E., Lascurain R., Montaño L. F., Vazquez L., Debray H., Montreuil J. Specificity of Amaranthus leucocarpus lectin. Glycoconj J. 1992 Aug;9(4):204–208. doi: 10.1007/BF00731166. [DOI] [PubMed] [Google Scholar]





