Abstract
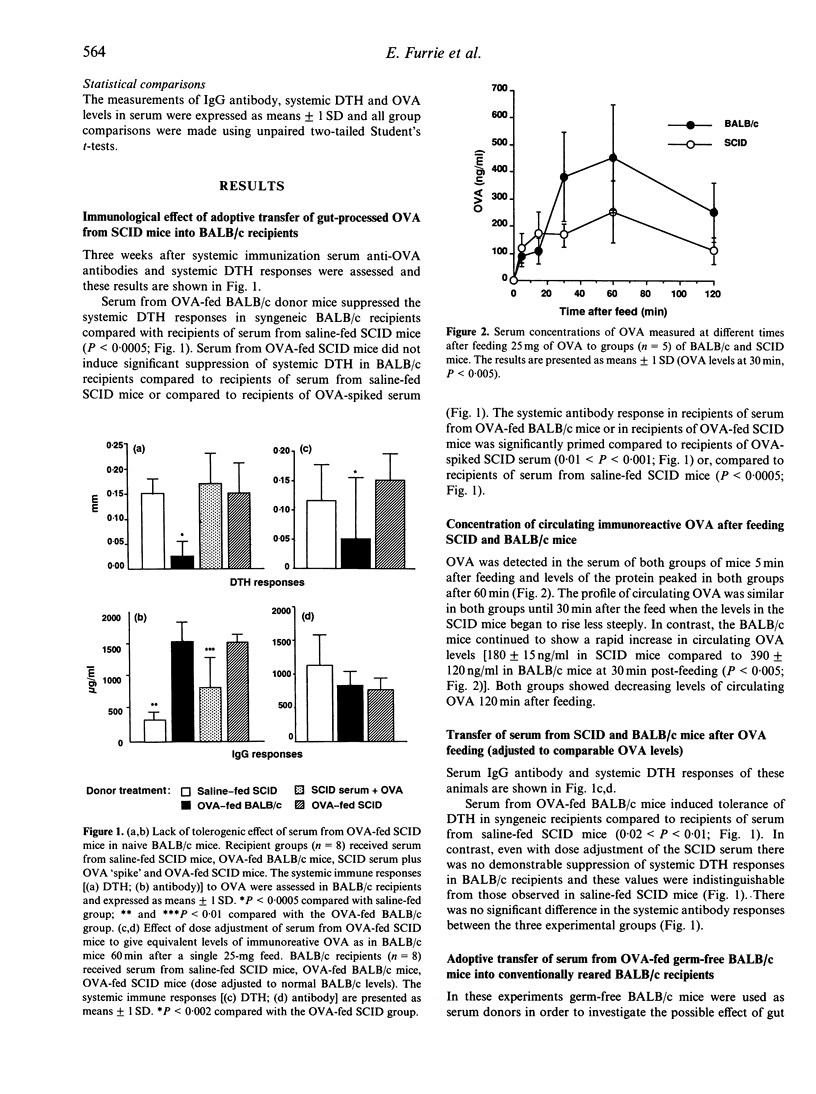
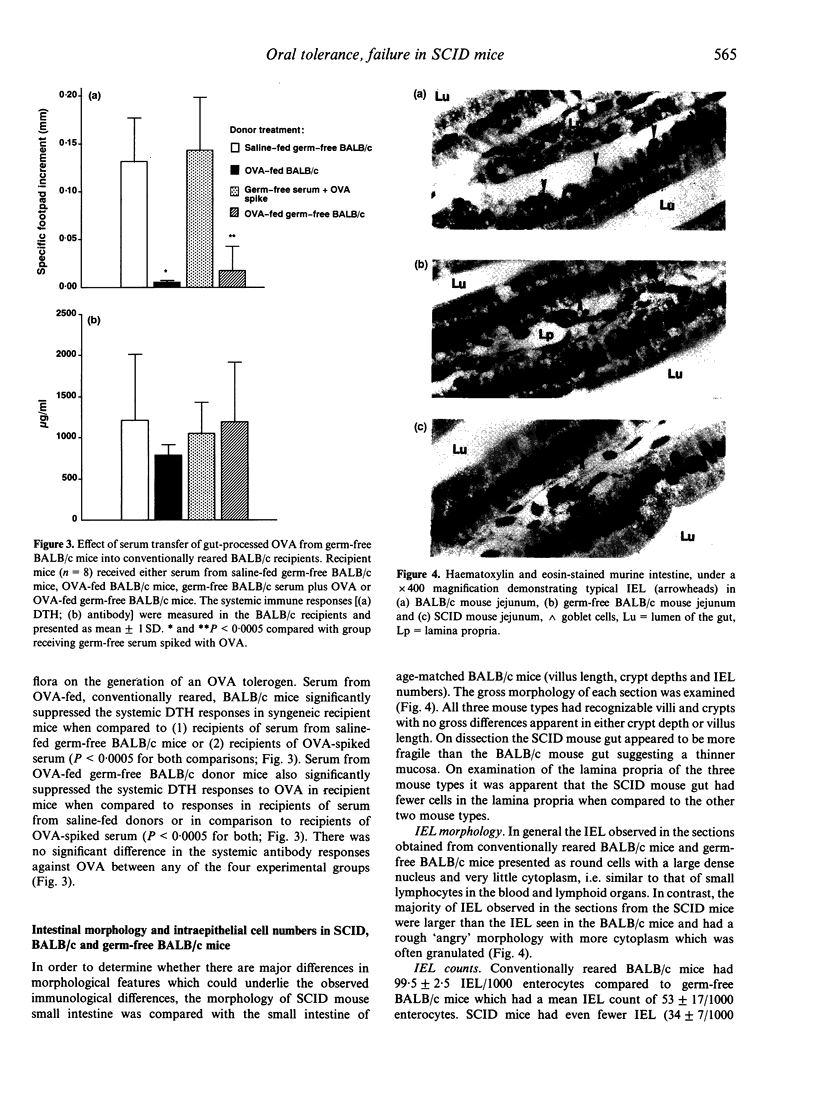
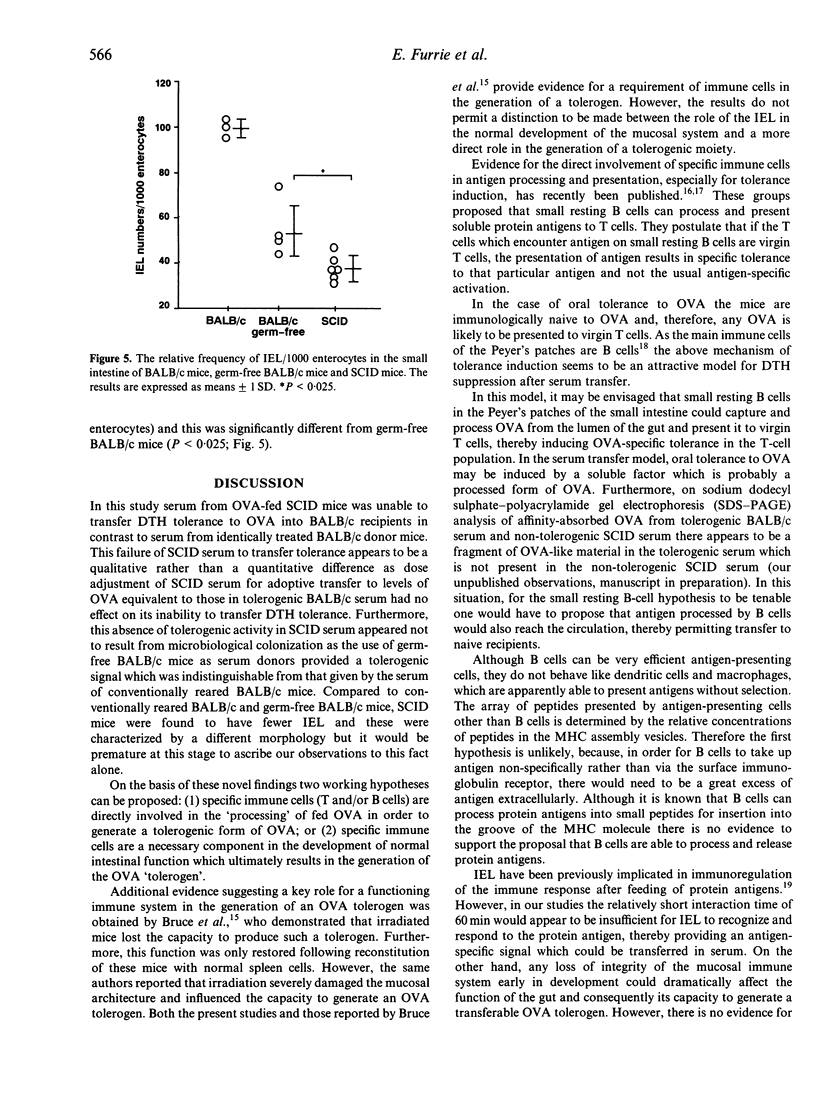
The role of the mucosal immune system in the generation of circulating tolerogenic ovalbumin (OVA) moieties has been investigated after a single feed of the protein. Serum collected from SCID mice 1 hr after a 25-mg feed of OVA was unable to transfer tolerance of delayed-type hypersensitivity (DTH) into naive BALB/c recipients. This is in contrast to serum collected from BALB/c mice which was able to transfer DTH tolerance to naive BALB/c recipients. The levels of circulating OVA detected in the serum of SCID mice 60 min after feeding OVA were approximately half those detected in the serum of BALB/c mice at the same time-point. However even dose adjustment of SCID mouse serum to a level of immunoreactive OVA equivalent to that found in BALB/c serum was unable to induce DTH tolerance in BALB/c recipients. This failure of SCID serum to transfer tolerance was shown to be unrelated to the germ-free conditions under which SCID mice are kept. Serum from OVA-fed germ-free BALB/c mice transferred DTH tolerance at equivalent levels to serum from conventionally reared BALB/c mice. When the intestinal morphology and intraepithelial lymphocyte (IEL) numbers in the duodenum of SCID mice were compared to conventionally reared and germ-free BALB/c controls, SCID mice were characterized by a lower number of IEL with a different morphology from the majority of IEL found in BALB/c mice.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Unanue E. R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991 Dec;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Barrett T. A., Gajewski T. F., Danielpour D., Chang E. B., Beagley K. W., Bluestone J. A. Differential function of intestinal intraepithelial lymphocyte subsets. J Immunol. 1992 Aug 15;149(4):1124–1130. [PubMed] [Google Scholar]
- Bland P. W., Whiting C. V. Antigen processing by isolated rat intestinal villus enterocytes. Immunology. 1989 Dec;68(4):497–502. [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bruce M. G., Ferguson A. Oral tolerance to ovalbumin in mice: studies of chemically modified and 'biologically filtered' antigen. Immunology. 1986 Apr;57(4):627–630. [PMC free article] [PubMed] [Google Scholar]
- Bruce M. G., Ferguson A. The influence of intestinal processing on the immunogenicity and molecular size of absorbed, circulating ovalbumin in mice. Immunology. 1986 Oct;59(2):295–300. [PMC free article] [PubMed] [Google Scholar]
- Bruce M. G., Strobel S., Hanson D. G., Ferguson A. Irradiated mice lose the capacity to 'process' fed antigen for systemic tolerance of delayed-type hypersensitivity. Clin Exp Immunol. 1987 Dec;70(3):611–618. [PMC free article] [PubMed] [Google Scholar]
- Croitoru K., Stead R. H., Bienenstock J., Fulop G., Harnish D. G., Shultz L. D., Jeffery P. K., Ernst P. B. Presence of intestinal intraepithelial lymphocytes in mice with severe combined immunodeficiency disease. Eur J Immunol. 1990 Mar;20(3):645–651. doi: 10.1002/eji.1830200327. [DOI] [PubMed] [Google Scholar]
- Custer R. P., Bosma G. C., Bosma M. J. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985 Sep;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- Ermak T. H., Owen R. L. Differential distribution of lymphocytes and accessory cells in mouse Peyer's patches. Anat Rec. 1986 Jun;215(2):144–152. doi: 10.1002/ar.1092150208. [DOI] [PubMed] [Google Scholar]
- Eynon E. E., Parker D. C. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992 Jan 1;175(1):131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut. 1971 Dec;12(12):988–994. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Hardy R. R., Bosma M. J. Transgenic scid mice with a functionally rearranged immunoglobulin heavy chain gene. Curr Top Microbiol Immunol. 1989;152:107–114. doi: 10.1007/978-3-642-74974-2_14. [DOI] [PubMed] [Google Scholar]
- Fuchs E. J., Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992 Nov 13;258(5085):1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- Hanson D. G., Vaz N. M., Maia L. C., Lynch J. M. Inhibition of specific immune responses by feeding protein antigens. III. Evidence against maintenance of tolerance to ovalbumin by orally induced antibodies. J Immunol. 1979 Nov;123(5):2337–2343. [PubMed] [Google Scholar]
- Kiyono H., Fujihashi K., Taguchi T., Aicher W. K., McGhee J. R. Regulatory functions for murine intraepithelial lymphocytes in mucosal responses. Immunol Res. 1991;10(3-4):324–330. doi: 10.1007/BF02919716. [DOI] [PubMed] [Google Scholar]
- Klein J. R. Ontogeny of the Thy-1-, Lyt-2+ murine intestinal intraepithelial lymphocyte. Characterization of a unique population of thymus-independent cytotoxic effector cells in the intestinal mucosa. J Exp Med. 1986 Jul 1;164(1):309–314. doi: 10.1084/jem.164.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987 Nov 1;166(5):1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H. J., Turner M. W., Strobel S. The generation of a 'tolerogen' after the ingestion of ovalbumin is time-dependent and unrelated to serum levels of immunoreactive antigen. Clin Exp Immunol. 1990 Sep;81(3):510–515. doi: 10.1111/j.1365-2249.1990.tb05365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Bosma M. J. Nature of the scid defect: a defective VDJ recombinase system. Curr Top Microbiol Immunol. 1989;152:55–62. doi: 10.1007/978-3-642-74974-2_8. [DOI] [PubMed] [Google Scholar]
- Strobel S., Mowat A. M., Drummond H. E., Pickering M. G., Ferguson A. Immunological responses to fed protein antigens in mice. II. Oral tolerance for CMI is due to activation of cyclophosphamide-sensitive cells by gut-processed antigen. Immunology. 1983 Jul;49(3):451–456. [PMC free article] [PubMed] [Google Scholar]
- Viney J. L., MacDonald T. T. Lymphokine secretion and proliferation of intraepithelial lymphocytes from murine small intestine. Immunology. 1992 Sep;77(1):19–24. [PMC free article] [PubMed] [Google Scholar]



