Abstract
Following a single percutaneous vaccination with optimally irradiated cercariae of Schistosoma mansoni, C57BL/6 mice mount a T-helper type-1 (Th1) lymphocyte-dominant immune response and are highly resistant to challenge infection. In this study, we show that, besides interferon-gamma (IFN-gamma), lymph node (LN) cells draining the site of vaccination produce significant amounts of interleukin (IL)-4 and IL-10 in culture with parasite antigen. After a challenge infection at the original site of vaccination, these LN cells did not generate an anamnestic Th1 response. Paradosically, IFN-gamma production and cell proliferation were profoundly down-regulated, whereas IL-4 production was enhanced and occurred earlier than in challenge control cultures. When challenge was applied to a site remote from vaccination, IFN-gamma down-regulation was less evident, but the IL-4 response was consistently enhanced. Neutralization of IL-10 in vitro restored IFN-gamma production by LN cells, whilst IL-4 levels were reduced. These data indicate that down-regulation of IFN-gamma is controlled by IL-10 and/or IL-4. Mice showing down-regulated Th1 responses in the LN after S. mansoni challenge infection did not have a reduced ability to eliminate challenge parasites, indicating that the post-vaccination Th1 response had already armed the lungs with effector T cells before administration of challenge parasites. The observed phenomena of down-regulated Th1 and enhanced Th2 responses may be of relevance to other systems involving multiple infections or vaccination/boosting. Repeated applications to percutaneous sites having common lymphatic drainage would be expected to favour Th2 responses. Alternatively, in order to induce Th1-dominant responses and avoid unwanted IL-4/IL-10 induction, the use of remote sites is indicated.
Full text
PDF

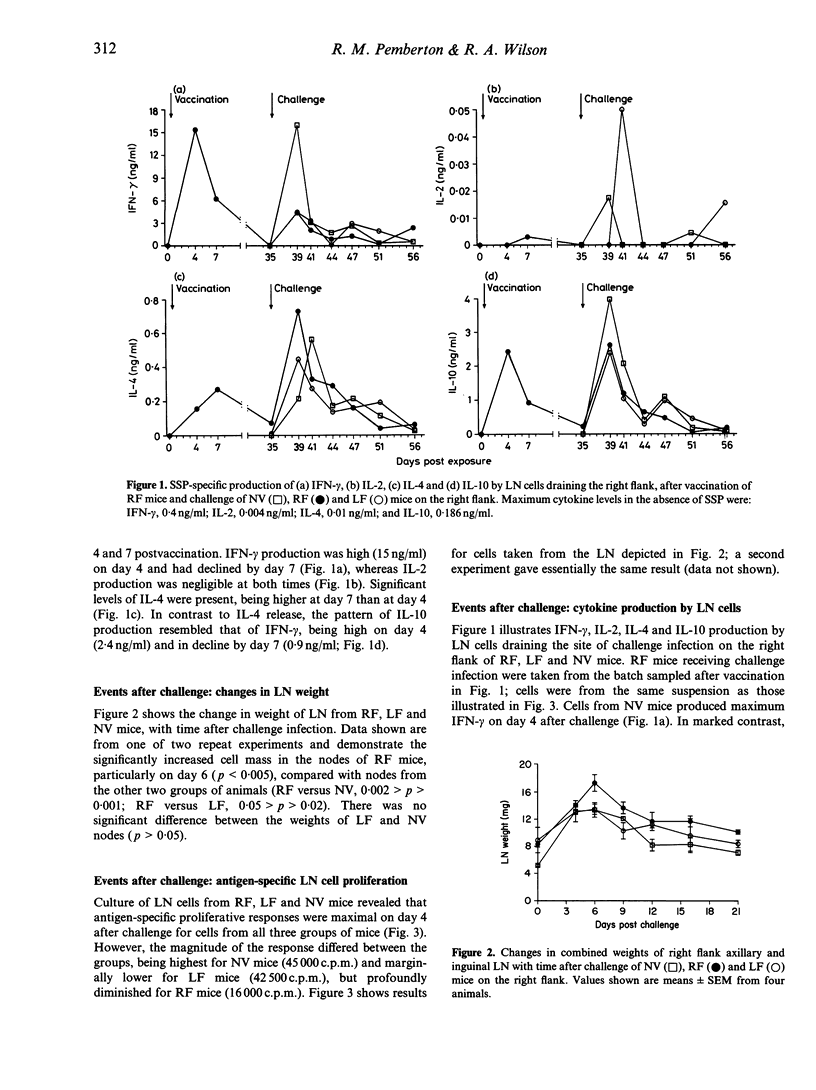
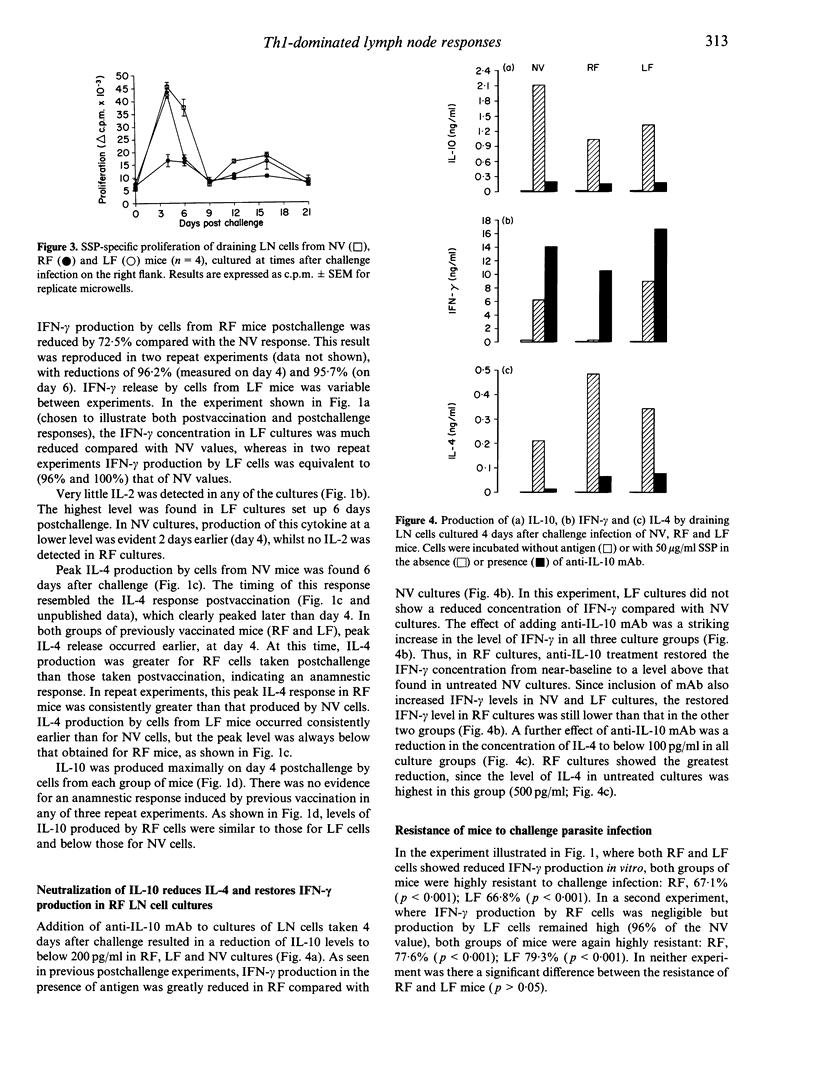



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken R., Coulson P. S., Dixon B., Wilson R. A. Radiation-resistant acquired immunity of vaccinated mice to Schistosoma mansoni. Am J Trop Med Hyg. 1987 Nov;37(3):570–577. doi: 10.4269/ajtmh.1987.37.570. [DOI] [PubMed] [Google Scholar]
- Burstein H. J., Shea C. M., Abbas A. K. Aqueous antigens induce in vivo tolerance selectively in IL-2- and IFN-gamma-producing (Th1) cells. J Immunol. 1992 Jun 15;148(12):3687–3691. [PubMed] [Google Scholar]
- Caulada-Benedetti Z., al-Zamel F., Sher A., James S. Comparison of Th1- and Th2-associated immune reactivities stimulated by single versus multiple vaccination of mice with irradiated Schistosoma mansoni cercariae. J Immunol. 1991 Mar 1;146(5):1655–1660. [PubMed] [Google Scholar]
- Constant S. L., Wilson R. A. In vivo lymphocyte responses in the draining lymph nodes of mice exposed to Schistosoma mansoni: preferential proliferation of T cells is central to the induction of protective immunity. Cell Immunol. 1992 Jan;139(1):145–161. doi: 10.1016/0008-8749(92)90108-2. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Wilson R. A. The role of pulmonary cellular reactions in the resistance of vaccinated mice to Schistosoma mansoni. Parasite Immunol. 1986 May;8(3):265–285. doi: 10.1111/j.1365-3024.1986.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Dean D. A. Schistosoma and related genera: acquired resistance in mice. Exp Parasitol. 1983 Feb;55(1):1–104. doi: 10.1016/0014-4894(83)90002-4. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Flores Villanueva P. O., Chikunguwo S. M., Harris T. S., Stadecker M. J. Role of IL-10 on antigen-presenting cell function for schistosomal egg-specific monoclonal T helper cell responses in vitro and in vivo. J Immunol. 1993 Sep 15;151(6):3192–3198. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. IV. Murine CTL clones produce IL-3 and GM-CSF, the activity of which is masked by the inhibitory action of secreted IFN-gamma. J Immunol. 1990 Jan 15;144(2):548–556. [PubMed] [Google Scholar]
- Gajewski T. F., Lancki D. W., Stack R., Fitch F. W. "Anergy" of TH0 helper T lymphocytes induces downregulation of TH1 characteristics and a transition to a TH2-like phenotype. J Exp Med. 1994 Feb 1;179(2):481–491. doi: 10.1084/jem.179.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Hsieh C. S., Heimberger A. B., Gold J. S., O'Garra A., Murphy K. M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E. A., Colley D. G. In vivo effects of monoclonal anti-L3T4 antibody on immune responsiveness of mice infected with Schistosoma mansoni. Reduction of irradiated cercariae-induced resistance. J Immunol. 1988 Apr 15;140(8):2737–2745. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Fiorentino D. F., Leverah J., Moore K. W., Bond M. W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990 Nov 1;145(9):2938–2945. [PubMed] [Google Scholar]
- Mountford A. P., Coulson P. S., Pemberton R. M., Smythies L. E., Wilson R. A. The generation of interferon-gamma-producing T lymphocytes in skin-draining lymph nodes, and their recruitment to the lungs, is associated with protective immunity to Schistosoma mansoni. Immunology. 1992 Feb;75(2):250–256. [PMC free article] [PubMed] [Google Scholar]
- Mountford A. P., Wilson R. A. Schistosoma mansoni: the effect of regional lymphadenectomy on the level of protection induced in mice by radiation-attenuated cercariae. Exp Parasitol. 1990 Nov;71(4):463–469. doi: 10.1016/0014-4894(90)90072-k. [DOI] [PubMed] [Google Scholar]
- Pemberton R. M., Smythies L. E., Mountford A. P., Wilson R. A. Patterns of cytokine production and proliferation by T lymphocytes differ in mice vaccinated or infected with Schistosoma mansoni. Immunology. 1991 Jul;73(3):327–333. [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Menon S., Coffman R. L. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993 Sep;23(9):2223–2229. doi: 10.1002/eji.1830230926. [DOI] [PubMed] [Google Scholar]
- Reiner S. L., Zheng S., Wang Z. E., Stowring L., Locksley R. M. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994 Feb 1;179(2):447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies L. E., Coulson P. S., Wilson R. A. Monoclonal antibody to IFN-gamma modifies pulmonary inflammatory responses and abrogates immunity to Schistosoma mansoni in mice vaccinated with attenuated cercariae. J Immunol. 1992 Dec 1;149(11):3654–3658. [PubMed] [Google Scholar]
- Smythies L. E., Pemberton R. M., Coulson P. S., Mountford A. P., Wilson R. A. T cell-derived cytokines associated with pulmonary immune mechanisms in mice vaccinated with irradiated cercariae of Schistosoma mansoni. J Immunol. 1992 Mar 1;148(5):1512–1518. [PubMed] [Google Scholar]
- Tanaka T., Hu-Li J., Seder R. A., Fazekas de St Groth B., Paul W. E. Interleukin 4 suppresses interleukin 2 and interferon gamma production by naive T cells stimulated by accessory cell-dependent receptor engagement. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5914–5918. doi: 10.1073/pnas.90.13.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velupillai P., Harn D. A. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome-infected mice: a mechanism for regulation of CD4+ T-cell subsets. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):18–22. doi: 10.1073/pnas.91.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali D. A., Bickle Q. D., Taylor M. G. Studies on immunity to Schistosoma mansoni in vivo: whole-body irradiation has no effect on vaccine-induced resistance in mice. Parasitology. 1988 Feb;96(Pt 1):49–61. doi: 10.1017/s0031182000081658. [DOI] [PubMed] [Google Scholar]
- Vignali D. A., Crocker P., Bickle Q. D., Cobbold S., Waldmann H., Taylor M. G. A role for CD4+ but not CD8+ T cells in immunity to Schistosoma mansoni induced by 20 krad-irradiated and Ro 11-3128-terminated infections. Immunology. 1989 Aug;67(4):466–472. [PMC free article] [PubMed] [Google Scholar]


