Abstract
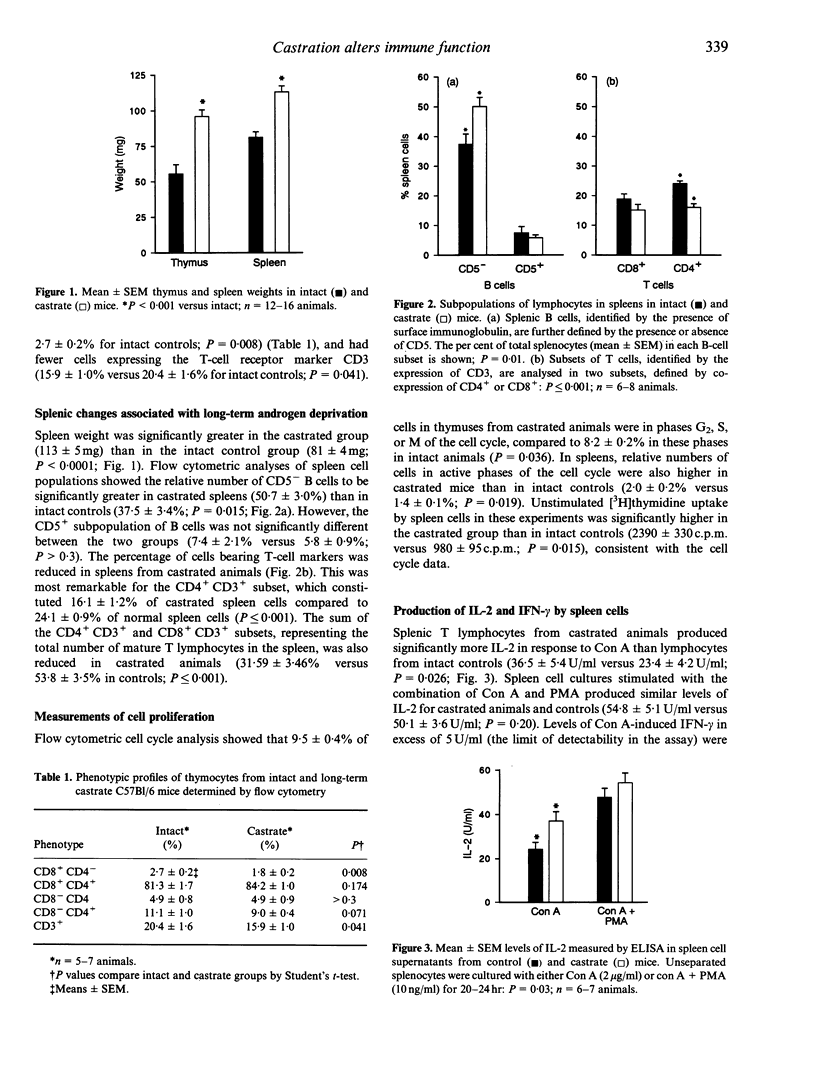
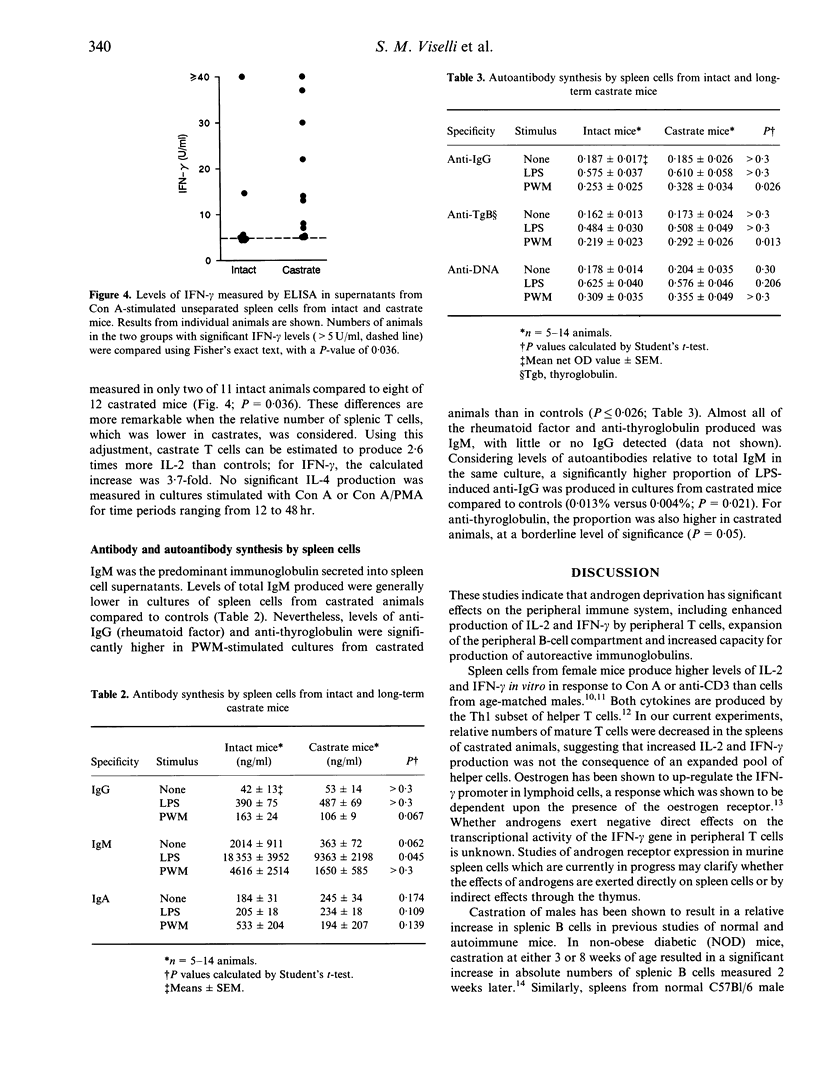
While it is generally recognized that females show enhanced cell-mediated and antibody responses to antigenic stimulation, the physiological basis for this observed sexual dimorphism of the immune response is not well understood. We report here studies on the effects of androgen deficiency on the peripheral immune system. Intact male mice were compared to animals castrated 3-4 months previously. Phenotypic characterization of thymocyte and lymphocyte subpopulations was carried out using dual-colour flow cytometry. In vitro production by spleen cells of interleukin-2 (IL-2), IL-4 and interferon-gamma (IFN-gamma), and levels of total immunoglobulin and autoreactive antibodies was measured by specific immunoassays. In addition to thymic hypertrophy, castrated animals showed significant splenic enlargement, which was largely owing to expansion of the B-cell population. The castrated spleens contained relatively fewer mature T cells than intact controls (P < or = 0.001), but culture supernatants from these spleen cells contained higher levels of IL-2 and IFN-gamma than control cultures (P < 0.04). Levels of in vitro antibody synthesis (IgM, IgG, IgA) were not higher in castrated animals compared to controls, but the castrate spleen cell cultures showed increased levels of production of two autoreactive antibodies, anti-IgG (rheumatoid factor) and anti-thyroglobulin. These data suggest that androgen deprivation results in a relative decrease in the number of mature peripheral T cells, but those which reach the spleen have functional characteristics suggestive of enhanced activation. Dysregulation in the B-cell compartment may be the result of altered effects of T-cell-mediated control.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansar Ahmed S., Dauphinée M. J., Montoya A. I., Talal N. Estrogen induces normal murine CD5+ B cells to produce autoantibodies. J Immunol. 1989 Apr 15;142(8):2647–2653. [PubMed] [Google Scholar]
- Ansar Ahmed S., Young P. R., Penhale W. J. Beneficial effect of testosterone in the treatment of chronic autoimmune thyroiditis in rats. J Immunol. 1986 Jan;136(1):143–147. [PubMed] [Google Scholar]
- Araneo B. A., Dowell T., Diegel M., Daynes R. A. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T cells. Blood. 1991 Aug 1;78(3):688–699. [PubMed] [Google Scholar]
- Batchelor J. R., Chapman B. A. The influence of sex upon the antibody response to an incompatible tumour. Immunology. 1965 Dec;9(6):553–564. [PMC free article] [PubMed] [Google Scholar]
- Brick J. E., Wilson D. A., Walker S. E. Hormonal modulation of responses to thymus-independent and thymus-dependent antigens in autoimmune NZB/W mice. J Immunol. 1985 Jun;134(6):3693–3698. [PubMed] [Google Scholar]
- DeFranco A. L. Molecular aspects of B-lymphocyte activation. Annu Rev Cell Biol. 1987;3:143–178. doi: 10.1146/annurev.cb.03.110187.001043. [DOI] [PubMed] [Google Scholar]
- Dunkel L., Taino V. M., Savilahti E., Eskola J. Effect of endogenous androgens on lymphocyte subpopulations. Lancet. 1985 Aug 24;2(8452):440–441. doi: 10.1016/s0140-6736(85)92755-2. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Garrett T. J. Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J Exp Med. 1972 Nov 1;136(5):1098–1116. doi: 10.1084/jem.136.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish F., Ziff M. A sensitive solid phase microradioimmunoassay for anti-double stranded DNA antibodies. Arthritis Rheum. 1981 Mar;24(3):534–543. doi: 10.1002/art.1780240314. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F., Lepault F., Homo-Delarche F., Bach J. F., Dardenne M. Influence of castration, alone or combined with thymectomy, on the development of diabetes in the nonobese diabetic mouse. Endocrinology. 1991 Sep;129(3):1382–1390. doi: 10.1210/endo-129-3-1382. [DOI] [PubMed] [Google Scholar]
- Fox H. S., Bond B. L., Parslow T. G. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991 Jun 15;146(12):4362–4367. [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKearn J. P., Paslay J. W., Slack J., Baum C., Davie J. M. B cell subsets and differential responses to mitogens. Immunol Rev. 1982;64:5–23. doi: 10.1111/j.1600-065x.1982.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Olsen N. J., Viselli S. M., Shults K., Stelzer G., Kovacs W. J. Induction of immature thymocyte proliferation after castration of normal male mice. Endocrinology. 1994 Jan;134(1):107–113. doi: 10.1210/endo.134.1.8275924. [DOI] [PubMed] [Google Scholar]
- Olsen N. J., Watson M. B., Henderson G. S., Kovacs W. J. Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinology. 1991 Nov;129(5):2471–2476. doi: 10.1210/endo-129-5-2471. [DOI] [PubMed] [Google Scholar]
- Paavonen T., Andersson L. C., Adlercreutz H. Sex hormone regulation of in vitro immune response. Estradiol enhances human B cell maturation via inhibition of suppressor T cells in pokeweed mitogen-stimulated cultures. J Exp Med. 1981 Dec 1;154(6):1935–1945. doi: 10.1084/jem.154.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'Homme G. J., Park C. L., Fieser T. M., Kofler R., Dixon F. J., Theofilopoulos A. N. Identification of a B cell differentiation factor(s) spontaneously produced by proliferating T cells in murine lupus strains of the lpr/lpr genotype. J Exp Med. 1983 Feb 1;157(2):730–742. doi: 10.1084/jem.157.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch P. S., Torres R. M., Engel D. Simultaneous cell cycle analysis and two-color surface immunofluorescence using 7-amino-actinomycin D and single laser excitation: applications to study of cell activation and the cell cycle of murine Ly-1 B cells. J Immunol. 1986 Apr 15;136(8):2769–2775. [PubMed] [Google Scholar]
- Reap E. A., Sobel E. S., Jennette J. C., Cohen P. L., Eisenberg R. A. Conventional B cells, not B1 cells, are the source of autoantibodies in chronic graft-versus-host disease. J Immunol. 1993 Dec 15;151(12):7316–7323. [PubMed] [Google Scholar]
- Souroujon M., White-Scharf M. E., Andreschwartz J., Gefter M. L., Schwartz R. S. Preferential autoantibody reactivity of the preimmune B cell repertoire in normal mice. J Immunol. 1988 Jun 15;140(12):4173–4179. [PubMed] [Google Scholar]
- Weinstein Y., Ran S., Segal S. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J Immunol. 1984 Feb;132(2):656–661. [PubMed] [Google Scholar]


