Abstract
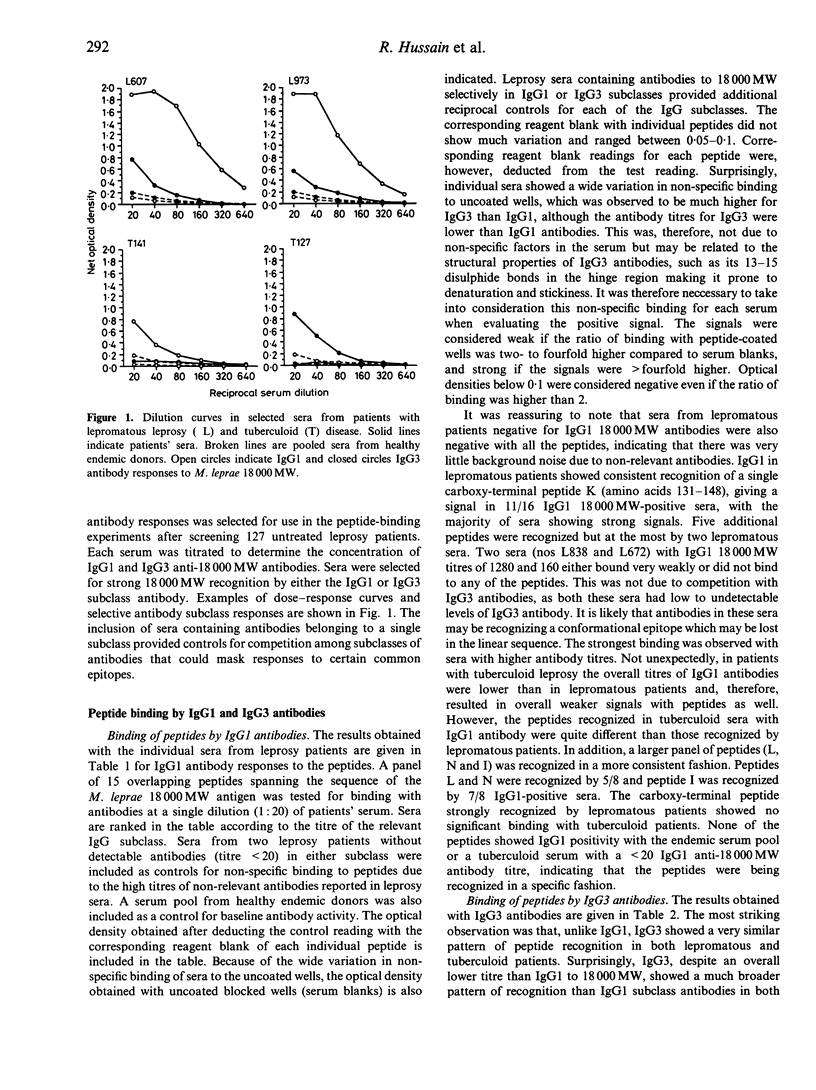
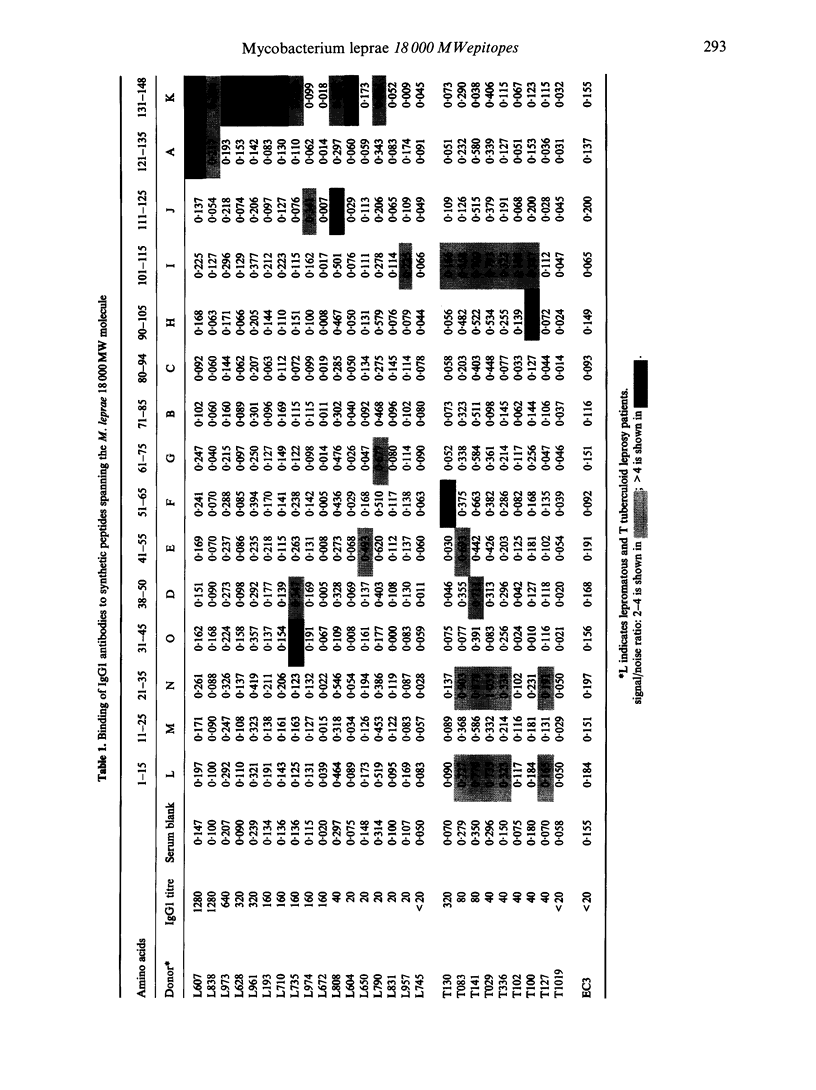
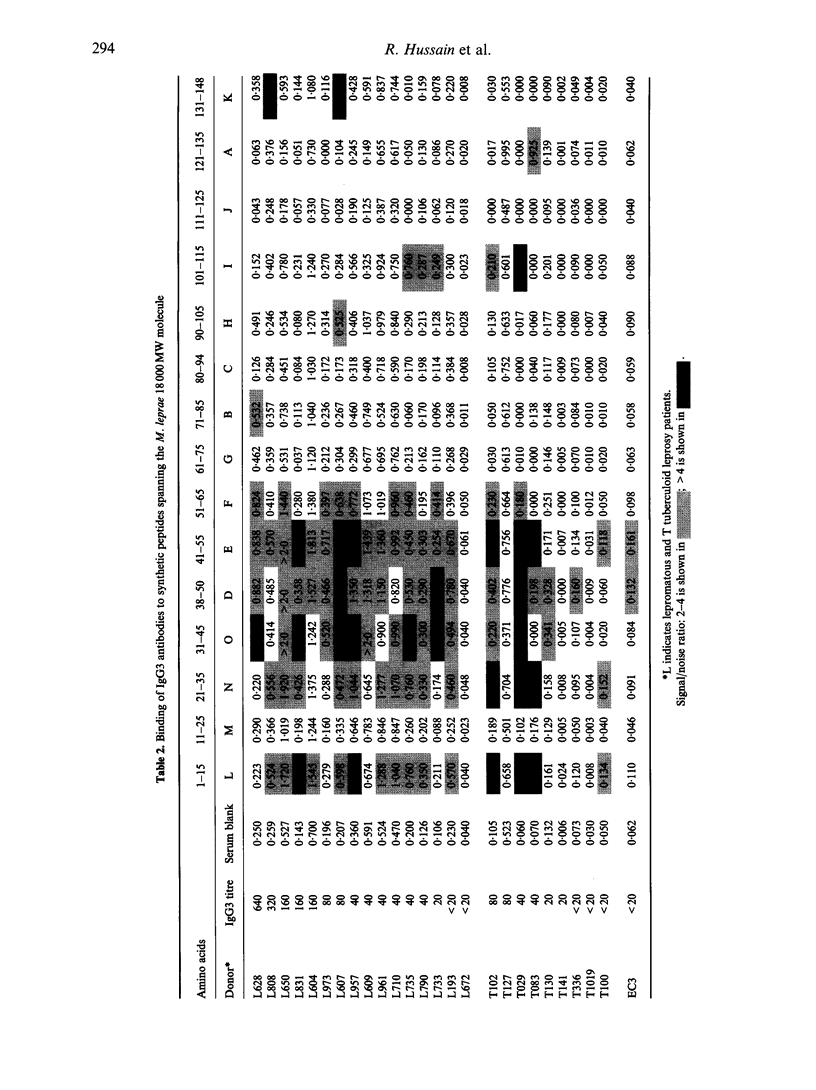
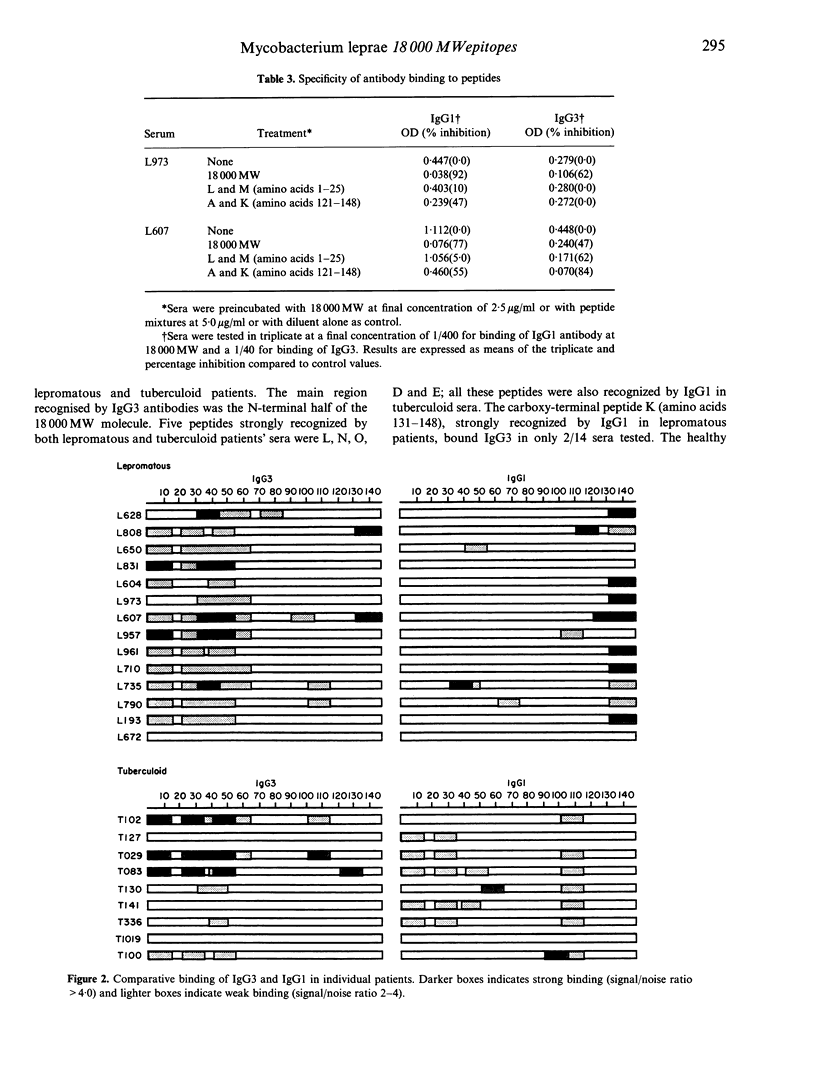
IgG subclasses are known to be differentially regulated by cytokines (elaborated by activated T cells), which act as growth factors and immunoglobulin switch factors on B cells. In leprosy, we have previously shown that IgG subclass antibodies to a purified recombinant antigen of Mycobacterium leprae (18,000 MW) are restricted to IgG1 and IgG3 across the disease spectrum. The only significant difference observed was that lepromatous patients with low to undetectable T-cell responses showed a strong correlation between IgG1 and IgG3 (P < 0.001) antibodies while tuberculoid patients who showed strong T-cell responses did not show such a correlation. To examine if these differences were related to T-cell-mediated class switching in tuberculoid leprosy patients, we have studied epitope recognition by IgG1 and IgG3 using a panel of synthetic peptides spanning the 18,000 MW molecule in an enzyme-linked immunosorbent assay (ELISA). In lepromatous patients there was little similarity in peptide recognition by IgG1 and IgG3, with IgG1 recognition being restricted to a single dominant carboxy-terminal peptide while the IgG3 antibodies recognized a diverse set of peptides in the N-terminal half of the 18,000 MW molecule. In tuberculoid patients both IgG1 and IgG3 antibody showed recognition of similar peptides in the N-terminal half of the 18,000 MW molecule. Our results therefore support the hypothesis that immunoglobulin class switching is occurring in tuberculoid but not in lepromatous patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth R. J., Harris D. P., Love J. M., Watson J. D. Antigenic proteins of Mycobacterium leprae. Complete sequence of the gene for the 18-kDa protein. J Immunol. 1988 Jan 15;140(2):597–601. [PubMed] [Google Scholar]
- Christmas S. E., Meager A. Production of interferon-gamma and tumour necrosis factor-alpha by human T-cell clones expressing different forms of the gamma delta receptor. Immunology. 1990 Dec;71(4):486–492. [PMC free article] [PubMed] [Google Scholar]
- Doherty T. M., Booth R. J., Love S. G., Gibson J. J., Harding D. R., Watson J. D. Characterization of an antibody-binding epitope from the 18-kDa protein on Mycobacterium leprae. J Immunol. 1989 Mar 1;142(5):1691–1695. [PubMed] [Google Scholar]
- Doherty T. M., Bäckström B. T., Prestidge R. L., Love S. G., Harding D. R., Watson J. D. Immune responses to the 18-kDa protein of Mycobacterium leprae. Similar B cell epitopes but different T cell epitopes seen by inbred strains of mice. J Immunol. 1991 Mar 15;146(6):1934–1940. [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Harris D. P., Bäckström B. T., Booth R. J., Love S. G., Harding D. R., Watson J. D. The mapping of epitopes of the 18-kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J Immunol. 1989 Sep 15;143(6):2006–2012. [PubMed] [Google Scholar]
- Hofker M. H., Walter M. A., Cox D. W. Complete physical map of the human immunoglobulin heavy chain constant region gene complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5567–5571. doi: 10.1073/pnas.86.14.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain R., Dockrell H. M., Chiang T. J. IgG subclass antibody to Mycobacterium leprae 18,000 MW antigen is restricted to IgG1 and IgG3 in leprosy. Immunology. 1994 Nov;83(3):495–500. [PMC free article] [PubMed] [Google Scholar]
- Hussain R., Dockrell H. M., Kifayet A., Daud A., Watson J. D., Chiang T. J., Stoker N. G. Recognition of Mycobacterium leprae recombinant 18-kDa proteins in leprosy. Int J Lepr Other Mycobact Dis. 1992 Sep;60(3):368–375. [PubMed] [Google Scholar]
- Hussain R., Poindexter R. W., Wistar R., Reimer C. B. Use of monoclonal antibodies to quantify subclasses of human IgG. I. Development of two-site immunoenzymometric assays for total IgG subclass determinations. J Immunol Methods. 1986 Oct 23;93(1):89–96. doi: 10.1016/0022-1759(86)90437-0. [DOI] [PubMed] [Google Scholar]
- Jefferis R., Reimer C. B., Skvaril F., de Lange G., Ling N. R., Lowe J., Walker M. R., Phillips D. J., Aloisio C. H., Wells T. W. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol Lett. 1985;10(3-4):223–252. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- Lamb F. I., Singh N. B., Colston M. J. The specific 18-kilodalton antigen of Mycobacterium leprae is present in Mycobacterium habana and functions as a heat-shock protein. J Immunol. 1990 Mar 1;144(5):1922–1925. [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- Perlmutter A. P., Gilbert W. Antibodies of the secondary response can be expressed without switch recombination in normal mouse B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7189–7193. doi: 10.1073/pnas.81.22.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Shimizu A., Honjo T. Synthesis and regulation of trans-mRNA encoding the immunoglobulin epsilon heavy chain. FASEB J. 1993 Jan;7(1):149–154. doi: 10.1096/fasebj.7.1.7916698. [DOI] [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]




