Abstract
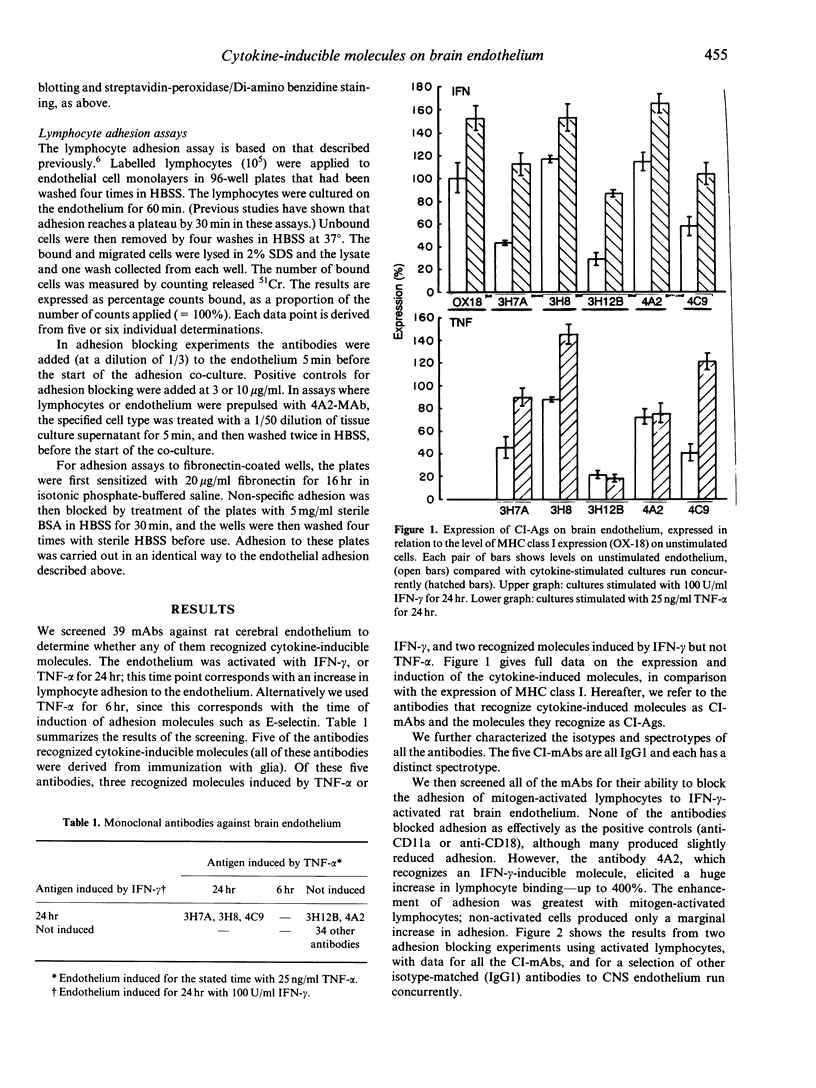
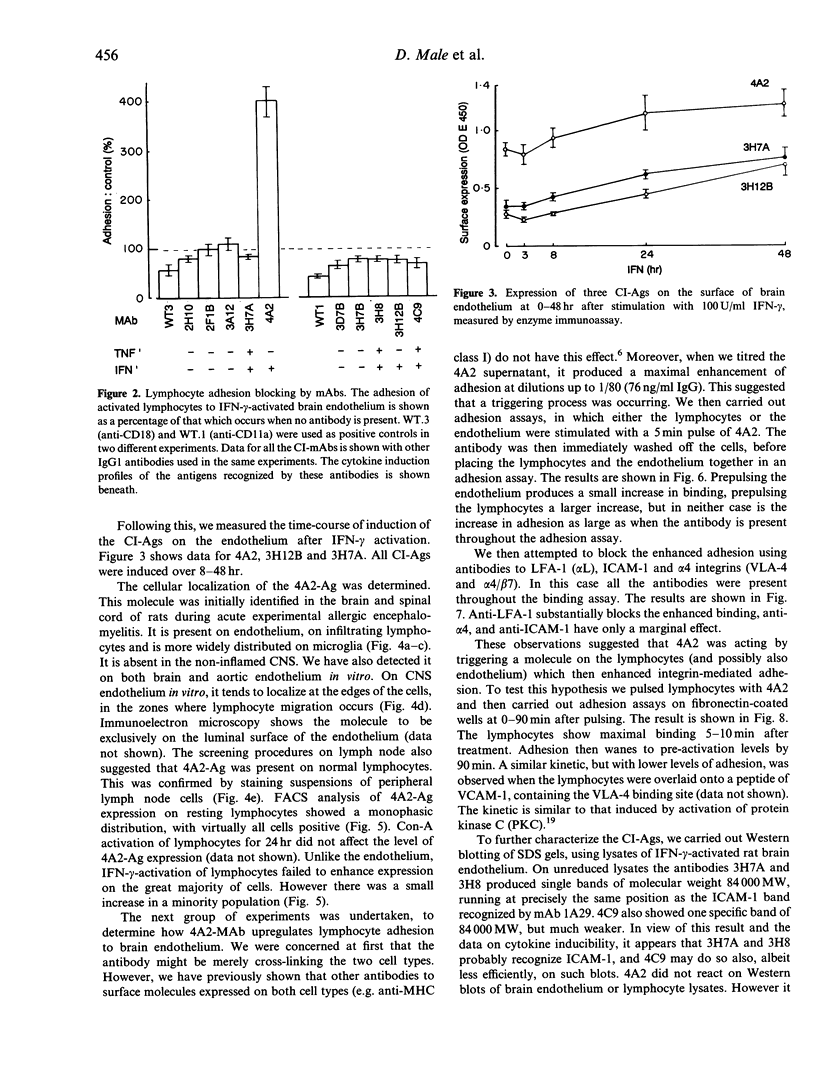
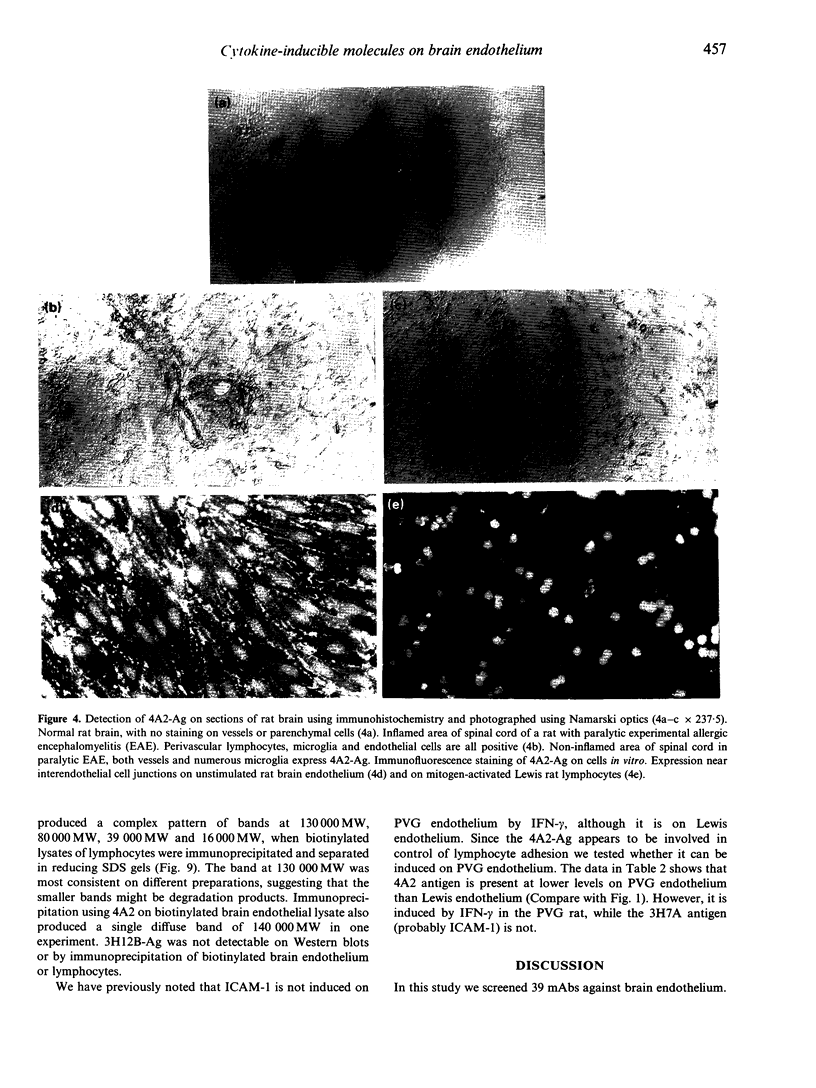
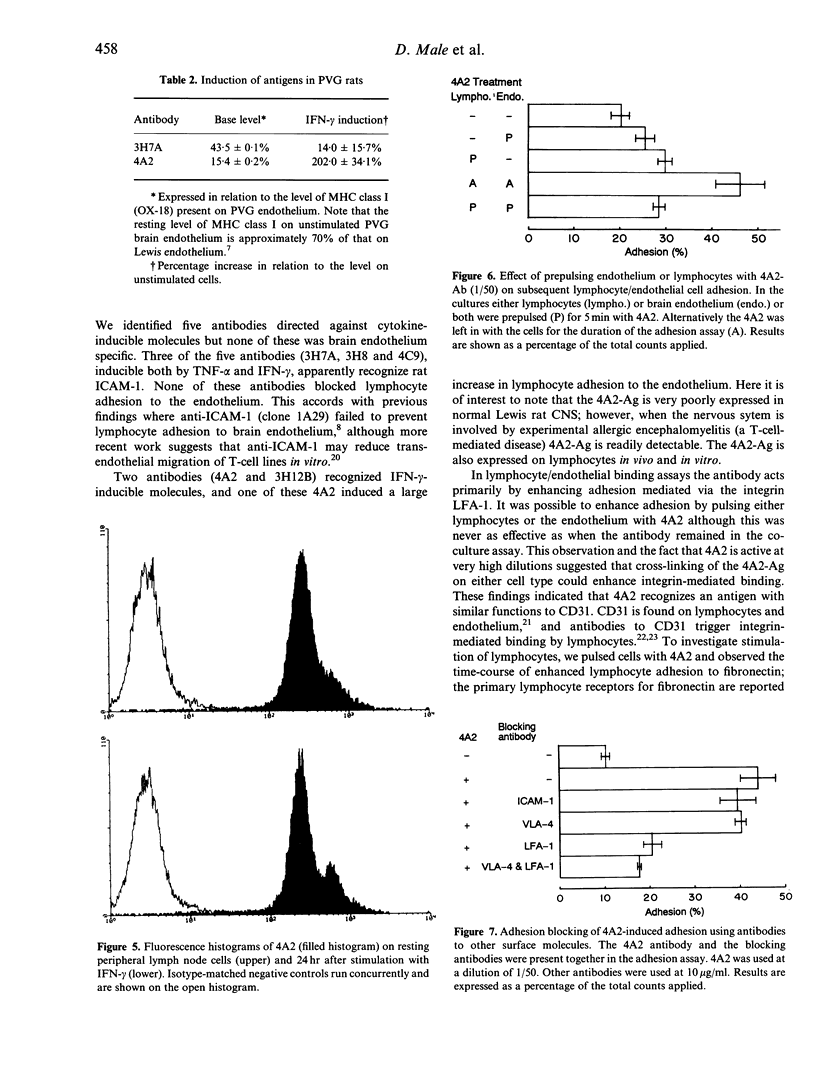
We undertook a search for cytokine-inducible molecules present on brain endothelium and which are involved in the control of lymphocyte adhesion. We screened 39 monoclonal antibodies (mAb) against rat brain endothelium in vitro, and identified five recognizing cytokine-inducible molecules. None of the 39 antibodies blocked lymphocyte adhesion, but one antibody (4A2), produced a 400% enhancement of lymphocyte binding. The 4A2 antigen is induced on brain endothelium by interferon-gamma (INF-gamma) but not tumour necrosis factor-alpha (TNF-alpha), at 6-48 hr. It is preferentially expressed near inter-endothelial cell junctions, but it also expressed on all lymphocytes and weakly on aortic endothelium in vitro. In vivo, it is not detectable on cells in the normal central nervous system (CNS), however it appears in the CNS during T-cell mediated immune reactions. Triggering of cells via this molecule enhances integrin-mediated adhesion of lymphocytes to brain endothelium, primarily via LFA-1. Unlike ICAM-1, 4A2 antigen is induced on endothelium of both Lewis and PVG strains. Although, it has some functional properties of human CD31, the 4A2 antigen is not rat CD31. The cellular localization of this molecule, its actions on integrin-mediated adhesion and its induction by IFN-gamma, all indicate that the 4A2 antibody recognizes a molecule involved in the control of lymphocyte migration into the brain.
Full text
PDF

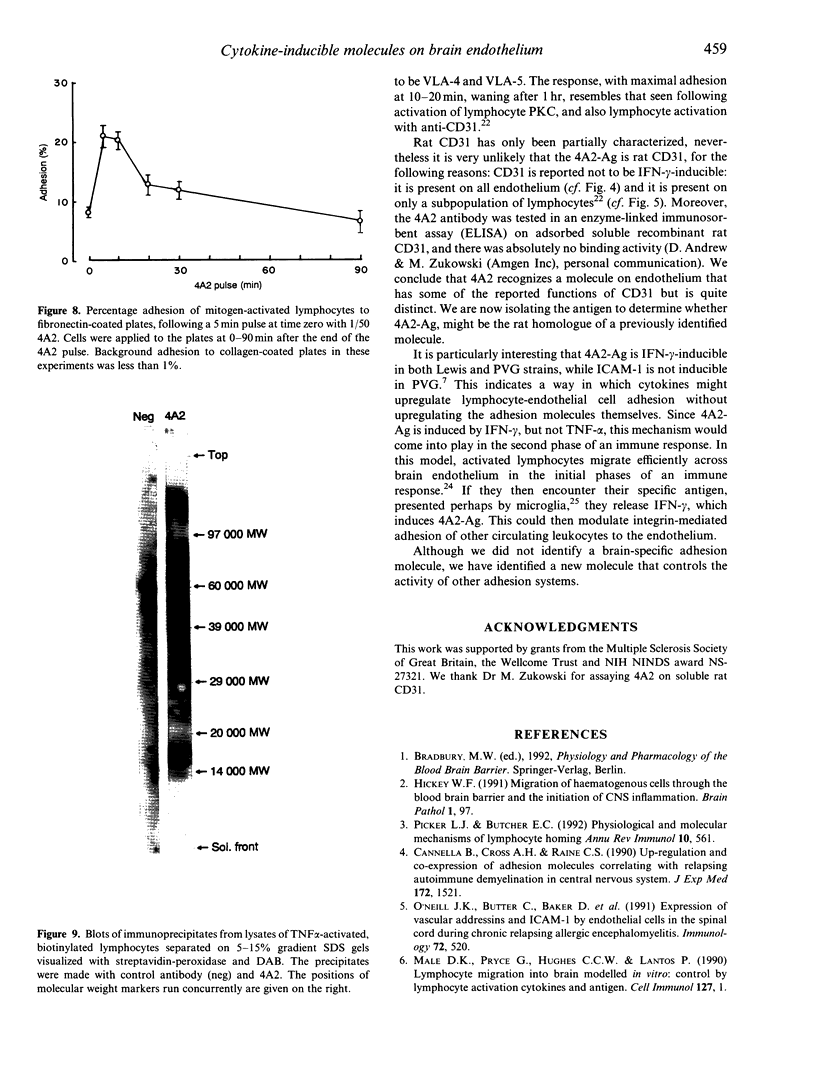

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Muller W. A., Buck C. A., Newman P. J. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991 Sep;114(5):1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman P. D., Ennis S. R., Rarey K. E., Betz A. L., Goldstein G. W. Brain microvessel endothelial cells in tissue culture: a model for study of blood-brain barrier permeability. Ann Neurol. 1983 Oct;14(4):396–402. doi: 10.1002/ana.410140403. [DOI] [PubMed] [Google Scholar]
- Cannella B., Cross A. H., Raine C. S. Upregulation and coexpression of adhesion molecules correlate with relapsing autoimmune demyelination in the central nervous system. J Exp Med. 1990 Nov 1;172(5):1521–1524. doi: 10.1084/jem.172.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. R., Ashman L. K., Ey P. L. Biotinylation: an alternative to radioiodination for the identification of cell surface antigens in immunoprecipitates. Mol Immunol. 1987 Jul;24(7):699–705. doi: 10.1016/0161-5890(87)90051-4. [DOI] [PubMed] [Google Scholar]
- Cross A. H., Cannella B., Brosnan C. F., Raine C. S. Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab Invest. 1990 Aug;63(2):162–170. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- Flaris N. A., Densmore T. L., Molleston M. C., Hickey W. F. Characterization of microglia and macrophages in the central nervous system of rats: definition of the differential expression of molecules using standard and novel monoclonal antibodies in normal CNS and in four models of parenchymal reaction. Glia. 1993 Jan;7(1):34–40. doi: 10.1002/glia.440070108. [DOI] [PubMed] [Google Scholar]
- Hickey W. F. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991 Jan;1(2):97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Linke A. T., Male D. K. Strain-specific variation in constitutive and inducible expression of MHC class II, class I and ICAM-1 on rat cerebral endothelium. Immunology. 1994 May;82(1):88–94. [PMC free article] [PubMed] [Google Scholar]
- Male D. K., Pryce G., Hughes C. C. Antigen presentation in brain: MHC induction on brain endothelium and astrocytes compared. Immunology. 1987 Mar;60(3):453–459. [PMC free article] [PubMed] [Google Scholar]
- Male D., Pryce G., Rahman J. Comparison of the immunological properties of rat cerebral and aortic endothelium. J Neuroimmunol. 1990 Dec;30(2-3):161–168. doi: 10.1016/0165-5728(90)90100-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Ohmori K., Fujiwara M. Immune regulation by brain cells in the central nervous system: microglia but not astrocytes present myelin basic protein to encephalitogenic T cells under in vivo-mimicking conditions. Immunology. 1992 Jun;76(2):209–216. [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Ratti C. M., McDonnell S. L., Cohn Z. A. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989 Aug 1;170(2):399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J. K., Butter C., Baker D., Gschmeissner S. E., Kraal G., Butcher E. C., Turk J. L. Expression of vascular addressins and ICAM-1 by endothelial cells in the spinal cord during chronic relapsing experimental allergic encephalomyelitis in the Biozzi AB/H mouse. Immunology. 1991 Apr;72(4):520–525. [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Bogue D. T., Ramberg J., Lipsky P. E. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991 Nov 1;147(9):2913–2921. [PubMed] [Google Scholar]
- Picker L. J., Butcher E. C. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Kotani M., Miyasaka M. Characterization of the rat leukocyte integrin, CD11/CD18, by the use of LFA-1 subunit-specific monoclonal antibodies. Eur J Immunol. 1991 Mar;21(3):627–633. doi: 10.1002/eji.1830210314. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Miyasaka M. Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int Immunol. 1990;2(2):165–171. doi: 10.1093/intimm/2.2.165. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Albelda S. M., Horgan K. J., van Seventer G. A., Shimizu Y., Newman W., Hallam J., Newman P. J., Buck C. A., Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992 Jul 1;176(1):245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock T. A., Cannon C., Fritz L. C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992 Mar 5;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]




