Abstract
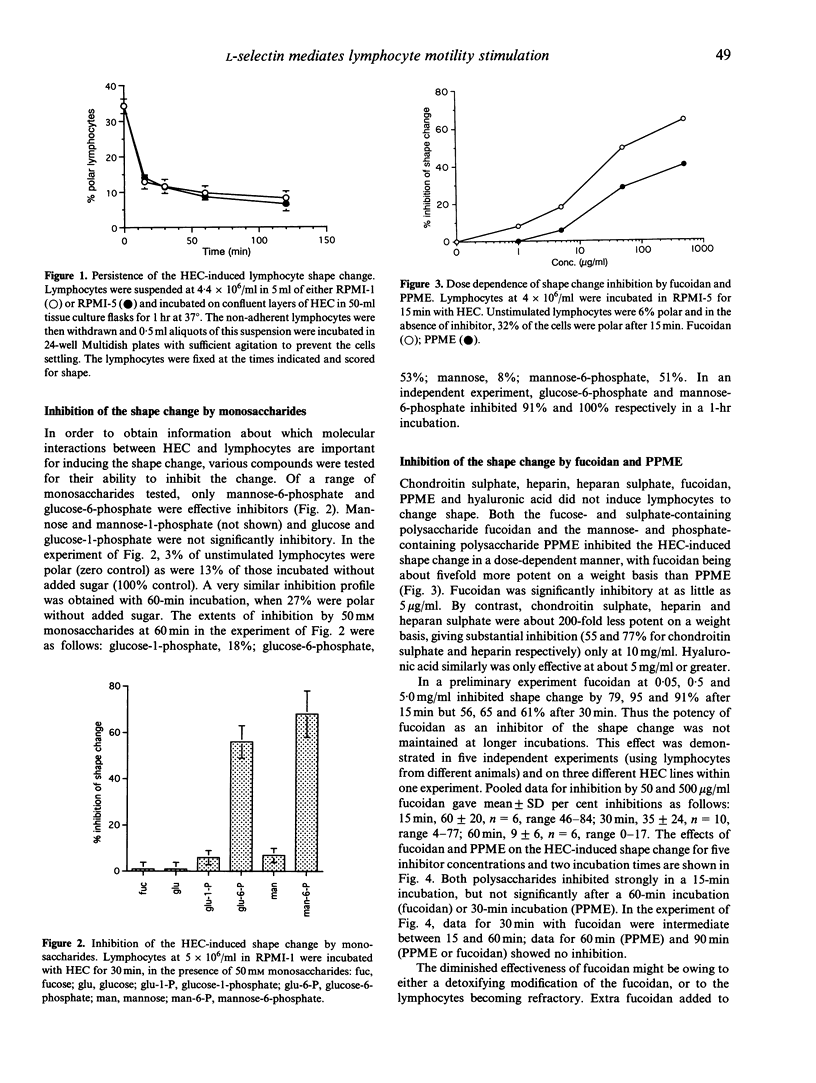
Lymphocyte emigration from blood into peripheral lymph nodes is mediated by specialized high endothelial cells (HEC) lining the postcapillary venules. A current model for this process postulates that it occurs in three steps: weak, selectin-mediated interactions tether lymphocytes to the blood vessel wall; the lymphocytes are activated to increase the affinity of integrin-dependent adhesion and enhance motility; and finally the lymphocytes migrate actively across the endothelial cell layer. Some features of this model are simulated in vitro by cultured HEC, which support the adhesion and transmigration of lymphocytes. In particular, cultured HEC stimulate lymphocytes to change shape from spherical to polar. This shape change provides a convenient assay of the motility activation of lymphocytes. In this paper it is shown that this occurs without the lymphocytes becoming tightly adherent, but depends on contact with the endothelial cell surface. The shape change is labile: non-adherent polar lymphocytes removed from HEC revert to round with a half-time of less than 8 min. Reagents which block the interaction of L-selectin with its ligands inhibit the HEC-induced shape change; these include mannose-6-phosphate, fucoidan, polyphosphomannan ester, treatment of HEC with sialidases and an anti-L-selectin monoclonal antibody known to block its lectin function. The change in shape is partially inhibited by antisera to the L-selectin ligand GlyCAM-1. Thus it is concluded that in this in vitro system, L-selectin-mediated binding of lymphocytes to HEC is essential for optimal induction of the shape change. Lymphocytes change shape in response to cultured HEC without loss of surface L-selectin, although activation stimuli are known to promote shedding of neutrophil L-selectin as well as motility and increased adhesiveness. However, the lymphocyte change in shape is a reversible process, and this may have implications for the nature and sequence of the signals transmitted from endothelium to lymphocytes during homing to peripheral lymph nodes.
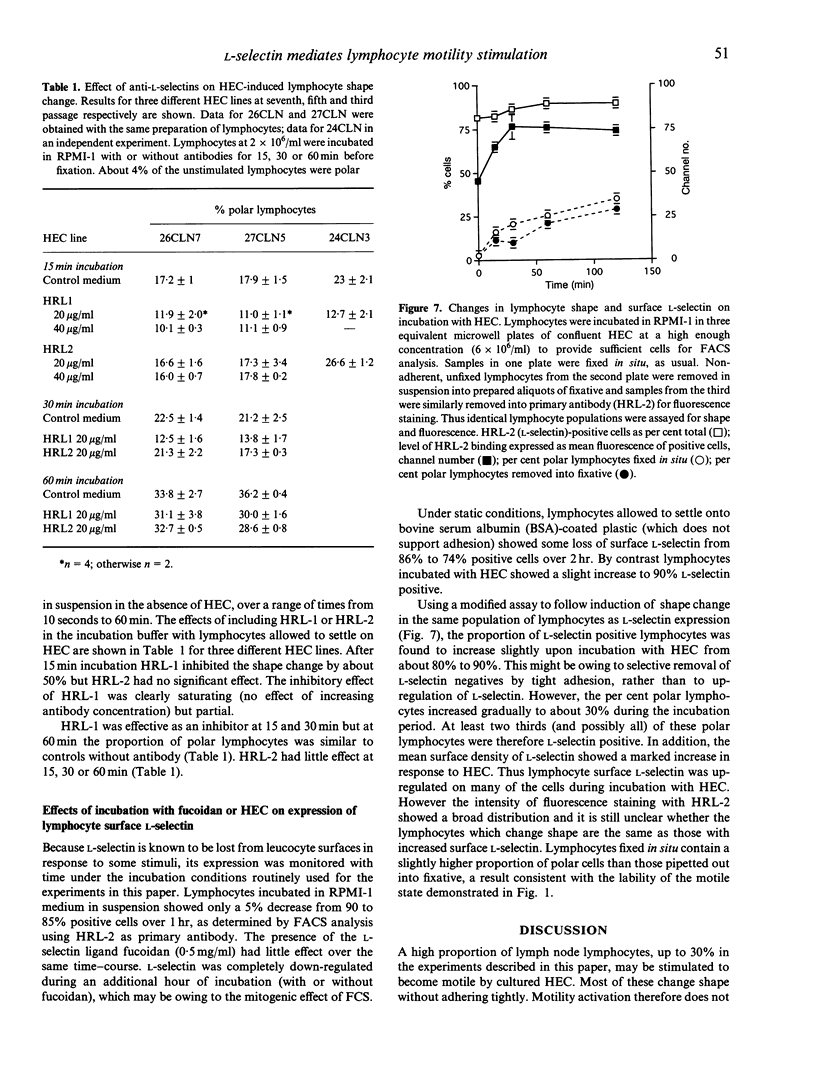
Full text
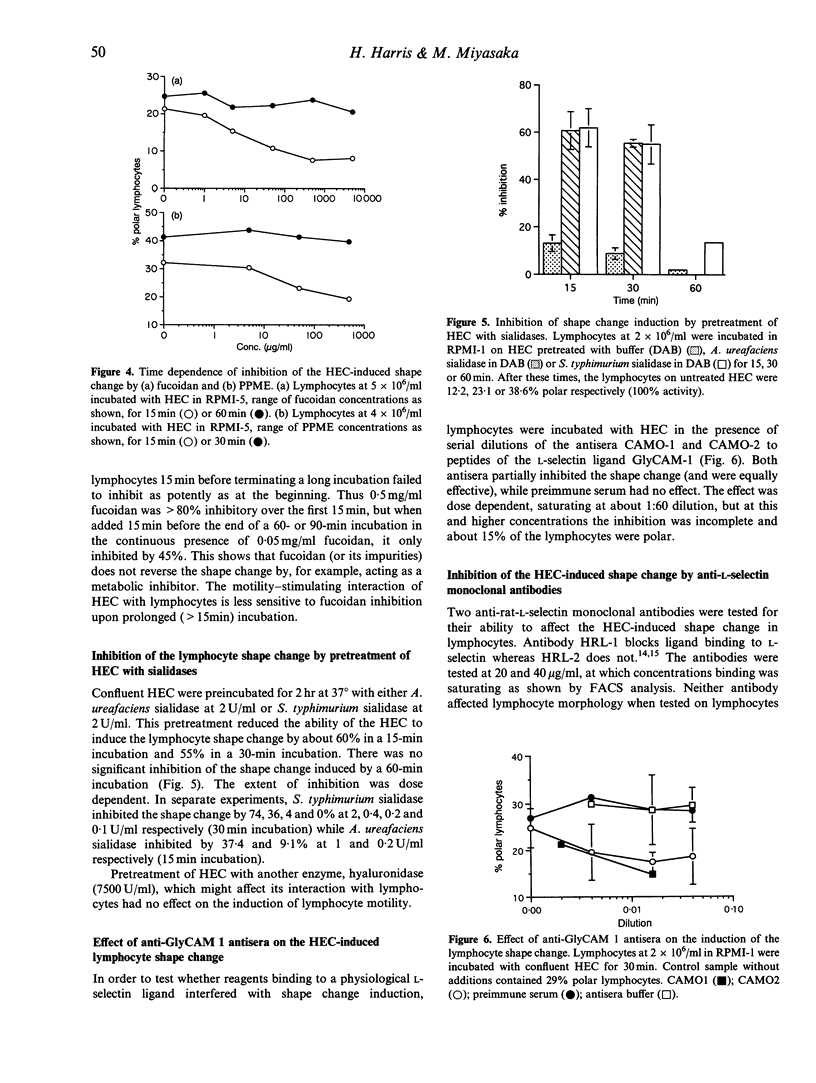
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A. Isolation and culture of high endothelial cells from rat lymph nodes. J Cell Sci. 1987 Feb;87(Pt 1):133–144. doi: 10.1242/jcs.87.1.133. [DOI] [PubMed] [Google Scholar]
- Ager A., Mistry S. Interaction between lymphocytes and cultured high endothelial cells: an in vitro model of lymphocyte migration across high endothelial venule endothelium. Eur J Immunol. 1988 Aug;18(8):1265–1274. doi: 10.1002/eji.1830180818. [DOI] [PubMed] [Google Scholar]
- Baumheter S., Singer M. S., Henzel W., Hemmerich S., Renz M., Rosen S. D., Lasky L. A. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993 Oct 15;262(5132):436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Robinson M. K., Warnock R. A., Butcher E. C. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991 Jul;114(2):343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandley B. K., Ross T. S., Schnaar R. L. Multiple carbohydrate receptors on lymphocytes revealed by adhesion to immobilized polysaccharides. J Cell Biol. 1987 Aug;105(2):991–997. doi: 10.1083/jcb.105.2.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Culty M., Miyake K., Kincade P. W., Sikorski E., Butcher E. C., Underhill C., Silorski E. The hyaluronate receptor is a member of the CD44 (H-CAM) family of cell surface glycoproteins. J Cell Biol. 1990 Dec;111(6 Pt 1):2765–2774. doi: 10.1083/jcb.111.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxall C., Watson S. R., Dowbenko D., Fennie C., Lasky L. A., Kiso M., Hasegawa A., Asa D., Brandley B. K. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J Cell Biol. 1992 May;117(4):895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Harris H. The stimulation of lymphocyte motility by cultured high endothelial cells and its inhibition by pertussis toxin. Int Immunol. 1991 Jun;3(6):535–542. doi: 10.1093/intimm/3.6.535. [DOI] [PubMed] [Google Scholar]
- Hourihan H., Allen T. D., Ager A. Lymphocyte migration across high endothelium is associated with increases in alpha 4 beta 1 integrin (VLA-4) affinity. J Cell Sci. 1993 Apr;104(Pt 4):1049–1059. doi: 10.1242/jcs.104.4.1049. [DOI] [PubMed] [Google Scholar]
- Imai Y., Lasky L. A., Rosen S. D. Further characterization of the interaction between L-selectin and its endothelial ligands. Glycobiology. 1992 Aug;2(4):373–381. doi: 10.1093/glycob/2.4.373. [DOI] [PubMed] [Google Scholar]
- Kansas G. S., Spertini O., Stoolman L. M., Tedder T. F. Molecular mapping of functional domains of the leukocyte receptor for endothelium, LAM-1. J Cell Biol. 1991 Jul;114(2):351–358. doi: 10.1083/jcb.114.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Butcher E. C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Dowbenko D., Imai Y., Henzel W. J., Grimley C., Fennie C., Gillett N., Watson S. R., Rosen S. D. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992 Jun 12;69(6):927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- Laudanna C., Constantin G., Baron P., Scarpini E., Scarlato G., Cabrini G., Dechecchi C., Rossi F., Cassatella M. A., Berton G. Sulfatides trigger increase of cytosolic free calcium and enhanced expression of tumor necrosis factor-alpha and interleukin-8 mRNA in human neutrophils. Evidence for a role of L-selectin as a signaling molecule. J Biol Chem. 1994 Feb 11;269(6):4021–4026. [PubMed] [Google Scholar]
- May M. J., Entwistle G., Humphries M. J., Ager A. VCAM-1 is a CS1 peptide-inhibitable adhesion molecule expressed by lymph node high endothelium. J Cell Sci. 1993 Sep;106(Pt 1):109–119. doi: 10.1242/jcs.106.1.109. [DOI] [PubMed] [Google Scholar]
- Mebius R. E., Watson S. R. L- and E-selectin can recognize the same naturally occurring ligands on high endothelial venules. J Immunol. 1993 Sep 15;151(6):3252–3260. [PubMed] [Google Scholar]
- Moore K. L., Varki A., McEver R. P. GMP-140 binds to a glycoprotein receptor on human neutrophils: evidence for a lectin-like interaction. J Cell Biol. 1991 Feb;112(3):491–499. doi: 10.1083/jcb.112.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecanda A., Walcheck B., Bishop D. K., Jutila M. A. Rapid activation-independent shedding of leukocyte L-selectin induced by cross-linking of the surface antigen. Eur J Immunol. 1992 May;22(5):1279–1286. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Butcher E. C. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Rosen S. D., Singer M. S., Yednock T. A., Stoolman L. M. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science. 1985 May 24;228(4702):1005–1007. doi: 10.1126/science.4001928. [DOI] [PubMed] [Google Scholar]
- Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992 Aug;13(8):291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Slodki M. E., Ward R. M., Boundy J. A. Concanavalin A as a probe of phosphomannan molecular structure. Biochim Biophys Acta. 1973 Apr 28;304(2):449–456. doi: 10.1016/0304-4165(73)90264-x. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stoolman L. M., Tenforde T. S., Rosen S. D. Phosphomannosyl receptors may participate in the adhesive interaction between lymphocytes and high endothelial venules. J Cell Biol. 1984 Oct;99(4 Pt 1):1535–1540. doi: 10.1083/jcb.99.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter P. R., Rouse B. T., Butcher E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988 Nov;107(5):1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekanecz Z., Humphries M. J., Ager A. Lymphocyte adhesion to high endothelium is mediated by two beta 1 integrin receptors for fibronectin, alpha 4 beta 1 and alpha 5 beta 1. J Cell Sci. 1992 Apr;101(Pt 4):885–894. doi: 10.1242/jcs.101.4.885. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Kitamura F., Kuida K., Shirao M., Mochizuki M., Suematsu M., Schmid-Schönbein G. W., Watanabe K., Tsurufuji S., Miyasaka M. Characterization of rat LECAM-1 (L-selectin) by the use of monoclonal antibodies and evidence for the presence of soluble LECAM-1 in rat sera. Eur J Immunol. 1993 Sep;23(9):2181–2188. doi: 10.1002/eji.1830230920. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Kuida K., Watanabe T., Koike S., Miyasaka M. Molecular mechanisms underlying lymphocyte recirculation. III. Characterization of the LECAM-1 (L-selectin)-dependent adhesion pathway in rats. J Immunol. 1993 Mar 1;150(5):1735–1745. [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H., Shaw S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol Today. 1993 Mar;14(3):111–115. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- True D. D., Singer M. S., Lasky L. A., Rosen S. D. Requirement for sialic acid on the endothelial ligand of a lymphocyte homing receptor. J Cell Biol. 1990 Dec;111(6 Pt 1):2757–2764. doi: 10.1083/jcb.111.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Imai Y., Fennie C., Geoffroy J. S., Rosen S. D., Lasky L. A. A homing receptor-IgG chimera as a probe for adhesive ligands of lymph node high endothelial venules. J Cell Biol. 1990 Jun;110(6):2221–2229. doi: 10.1083/jcb.110.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C. The locomotor capacity of human lymphocytes and its enhancement by cell growth. Immunology. 1986 Feb;57(2):281–289. [PMC free article] [PubMed] [Google Scholar]
- Yednock T. A., Stoolman L. M., Rosen S. D. Phosphomannosyl-derivatized beads detect a receptor involved in lymphocyte homing. J Cell Biol. 1987 Mar;104(3):713–723. doi: 10.1083/jcb.104.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk W., Brons N. H., Rozing J. Scanning electron microscopy of homing and recirculating lymphocyte populations. Cell Immunol. 1975 Oct;19(2):245–261. doi: 10.1016/0008-8749(75)90207-5. [DOI] [PubMed] [Google Scholar]


