Abstract
Regulated exocytosis is thought to occur either by “full fusion,” where the secretory vesicle fuses with the plasma membrane (PM) via a fusion pore that then dilates until the secretory vesicle collapses into the PM; or by “kiss-and-run,” where the fusion pore does not dilate and instead rapidly reseals such that the secretory vesicle is retrieved almost fully intact. Here, we describe growing evidence for a third form of exocytosis, dubbed “kiss-and-coat,” which is characteristic of a broad variety of cell types that undergo regulated exocytosis. Kiss-and-coat exocytosis entails prolonged maintenance of a dilated fusion pore and assembly of actin filament (F-actin) coats around the exocytosing secretory vesicles followed by direct retrieval of some fraction of the emptied vesicle membrane. We propose that assembly of the actin coats results from the union of the secretory vesicle membrane and PM and that this compartment mixing represents a general mechanism for generating local signals via directed membrane fusion.
INTRODUCTION
Exocytosis is the major mechanism for depositing material outside of cells, and, as such, is critically important for an enormous number of basic biological processes. Exocytosis is generally viewed as being either regulated or constitutive. Regulated exocytosis occurs in response to a specific stimulus, usually elevation of intracellular free calcium, whereas constitutive exocytosis occurs constantly and serves to maintain the normal complement of plasma membrane (PM) proteins and lipids. Regulated exocytosis is most frequently associated with neurotransmitter release at the synapse, but it is essential for many other processes, including fertilization, digestion, and immune and pulmonary system function, to name a few. Indeed, it has recently become apparent that virtually all cells can undergo regulated exocytosis in response to elevation of calcium resulting from cell damage and other insults (Reddy et al., 2001; McNeil and Steinhardt, 2003).
Two forms of regulated exocytosis have been described, based on the fate of the secretory vesicle membrane after fusion with the PM—“full fusion” and “kiss-and-run” (Fesce et al., 1994; Artalejo et al., 1998; Schneider, 2001; Valtorta et al., 2001; Wightman and Haynes, 2004). During full fusion, the fusion pore opens and dilates until the membrane of the secretory vesicle is completely collapsed into the PM. The excess membrane is subsequently retrieved by clathrin-dependent or -independent mechanisms (Figure 1; Table 1). During kiss-and-run, the fusion pore opens but does not dilate, maintaining a diameter of 1–5 nm (Lollike et al., 1995; Staal et al., 2004; Table 1), and a fraction of the vesicle contents are released. The fusion pore is then rapidly resealed, thereby retrieving the secretory vesicle membrane more or less intact (Figure 1). In both cases, the exocytotic event itself is quite rapid, typically occurring in a less than a second (Table 1).
Figure 1.
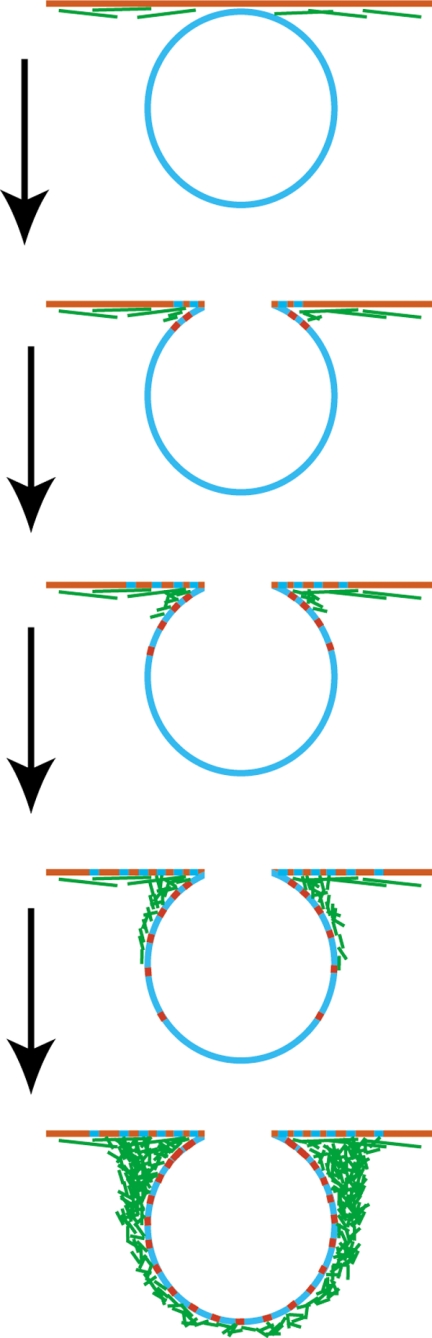
Schematic diagram showing different modes of exo/endocytosis. During full fusion, secretory vesicles fuse with the PM, undergo complete fusion pore dilation, and thereby collapse into the PM. The excess membrane is subsequently retrieved by clathrin-dependent and/or -independent endocytosis. During kiss-and-run, the secretory vesicle transiently fuses with the PM, maintaining a small fusion pore that then very rapidly reseals to effect retrieval. During kiss-and-coat, however, secretory vesicles fuse with the PM, undergo fusion pore dilation, and remain as morphologically distinct vesicle “ghosts” for many seconds to many minutes before fusion pore resealing and retrieval.
Table 1.
Features of different types of exo/endocytosis
| Cell type | Granule diameter (nm) | Granule contents | Fusion durationa | Pore diameter (nm) | F-actin coatb |
|---|---|---|---|---|---|
| Full fusion | |||||
| Frog neuromuscular junction | 50c | Acetylcholine | |||
| Hippocampal neuron | 50d | Glutamate | |||
| Midbrain neuro | 40-60e | Dopamine | |||
| Kiss-and-run | |||||
| Midbrain neuron | 40-50e | Dopamine | 200-300 μsf | 1.5-3.5f | |
| Hippocampal neuron | 50d | Glutamate | 400-860 msg | 1h | |
| <3 sh | |||||
| Posterior pituitary nerve terminal | 160i | 0.56 sj | 1j | ||
| Posterior pituitary nerve terminal | 50i | 0.31 sj | 0.3j | ||
| PC12 cell | 55k | <2 sk | 1.6k | ||
| Bovine chromaffin cell | 250-300l,m | Catecholamine | 0.3 to <5 sn,o | <3o | |
| Hippocampal astrocyte | 310p | Glutamate | 2 msp | ||
| Kiss-and-coat | |||||
| Frog egg | 500-3000q | Lectin | 10-60 sr | 500-1000s | Yesr,1,2 |
| Fish egg | 1,000-15,000t | Lectin | >56 st | 500-1000t | Yest,3 |
| Pancreatic acinar cell | 100-1,000u | Amylase | 30 s—4 minv,w | 200x | Yesy,z,2 |
| Parotid acinar cell | 200-5,500aa | Amylase | 40 s to >3.5 minaa | N.D. | |
| Alveolar type II cell | 700-3,000bb | Surfactant | 30 s—30 minbb,cc | 400bb,dd | Yesee,3 |
N.D., not determined.
Not shown for full fusion because the event is irreversible.
1, detection of actin coating by live imaging; 2, detection of actin coating by phalloidin staining in presence of fluorescent dextran as marker for vesicles that have exocytosed; and 3, phalloidin staining alone.
Heuser and Reese (1973); d Murthy et al. (2001); e Pothos et al. (2000); f Staal et al. (2004); g Ghandi and Stevens (2003); h Richards et al. (2005); i Morris and Nordmann (1980); j Klyachko and Jackson (2002); k Liu et al. (2005); l Neher and Zucker (1993); m Henkel et al. (2001); n Artalejo et al. (1995); o Albillos et al. (1997); p Chen et al. (2005a); q Campanella and Andreucetti (1977); r Sokac et al. (2003); s Boyle et al. (2001); t Becker and Hart (1999); u Schneider et al. (1997); v Nemoto et al. (2001); w Thorn et al. (2004); x Jena et al. (2003); y Nemoto et al. (2004); z Turvey and Thorn (2004); aa Chen et al. (2005b); bb Haller et al. (2001); cc Haller et al. (1998); dd Kliewer et al. (1985); ee van Weeren et al. (2004).
KISS-AND-COAT EXOCYTOSIS
It is now clear that both full fusion and kiss-and-run occur to varying extents in neurons and neuroendocrine cells that undergo exocytosis with very rapid kinetics (Klyachko and Jackson, 2002; Gandhi and Stevens, 2003; Allersma et al., 2004). Indeed, the relative fraction of exocytotic events that correspond to full fusion or kiss-and-run can be modulated within a given cell type by specific molecular interventions (Burgoyne and Barclay, 2002; Wang et al., 2003). However, it is also becoming apparent that several diverse cell types undergo regulated exocytosis in a far more sedate manner that does not conform to either full fusion or kiss-and-run (Table 1). Specifically, after fusion with the PM, cortical granules in eggs of sea urchins (Whalley et al., 1995), fish (Becker and Hart, 1999), and amphibians (Bement et al., 2000) are apparent as distinct compartments beneath the PM for ∼5–60 s or more (Table 1). Similarly, pancreatic acinar cell secretory zymogen granules can remain in a fused state with the PM from 30 s up to 15 min (Nemoto et al., 2001, 2004; Thorn et al., 2004; Thorn and Parker, 2005). Even more protracted is exocytosis in alveolar type II cells, where the fused lamellar body granules may persist as morphologically distinct compartments for 30 min or more (Haller et al., 1998, 2001). And, in all of the above-mentioned cases, the fused vesicles have fusion pores 2–3 orders of magnitude larger than the ∼1- to 5-nm pores calculated for kiss-and-run exocytosis (Table 1). The ultimate fate of the secretory vesicle membrane remnants in these cell types is not as well understood as during full fusion or kiss-and-run, but at least some of it is apparently retrieved without collapsing into the PM (see below).
The observation that the emptied secretory vesicle persists as a morphologically distinct compartment beneath the PM for many seconds to many minutes differentiates this type of exocytosis from full fusion, whereas the dilation and maintenance of the fusion pore also render it distinct from kiss-and-run. Thus, a third type of exo/endocytosis exists, used by otherwise diverse cell types that undergo regulated secretion. Because this type of exocytosis is associated with assembly of actin coats around the exocytosing secretory vesicles (see below), we suggest the name “kiss-and-coat” to distinguish this mode of exo/endocytosis from full fusion and kiss-and-run.
Identification of kiss-and-coat as a distinct mechanism in regulated exocytosis is important for at least four reasons. First, it is likely that kiss-and-coat exocytosis is uniquely adapted to promote discharge of secretory material under conditions where it cannot be released rapidly. That is, in the cell types most clearly shown to undergo kiss-and-coat exocytosis (Table 1), the secretory vesicles are much larger than those in many neurons and neuroendocrine cell types (500–15,000 versus 20–50 nm in diameter; Table 1), whereas the vesicle contents are often much less soluble. For example, egg cortical granules contain virtually crystalline arrays of lectins (Wessel et al., 2001), and lamellar bodies, the major secretory compartment in alveolar type II cells, contain tightly packed surfactant, which is comprised of a protein–phospholipid mixture (Dietl and Haller, 2005). So insoluble is surfactant, it frequently remains associated in a clot extending outside the cell from the fusion pore for many minutes after the onset of exocytosis (Haller et al., 2001). Extended fusion and dilated fusion pores would ensure complete release of such large macromolecules from large vesicles by maximizing the size and duration of the interface between the vesicle contents and the extracellular medium. Second, kiss-and-coat permits sequential exocytosis (Nemoto et al., 2001; Thorn and Parker, 2005). That is, by maintaining a relatively large, stable portal between the primary vesicle (i.e., the vesicle that first fuses with the PM), subsequent homotypic fusion events of secondary and tertiary vesicles are ensured of free movement of vesicle contents to the extracellular medium (Figure 1). Third, given the wealth of cell types with large secretory vesicles and the apparent frequency of sequential exocytosis, it is likely that kiss-and-coat is at least as common as either full fusion or kiss-and-run. Fourth, prolonged union of secretory vesicles with the PM without collapse of the vesicle membrane into the PM has critical mechanistic implications for the events that follow fusion. These implications are discussed next.
ACTIN COATING AS A SECRETORY VESICLE STABILIZER
A critical consequence of the above-mentioned examples of exocytosis is the creation of an inward extension of the PM that persists for many seconds to many minutes. In a sense, this represents an abeyance of compensatory endocytosis and must therefore be dealt with in a manner that ensures maintenance of the structural integrity of the PM. How is this accomplished? Live cell imaging of cortical granule exocytosis in Xenopus eggs (Sokac et al., 2003) has revealed that cortical granules become surrounded with F-actin after fusion with the PM (Figure 2). Such actin coats are not restricted to Xenopus eggs: F-actin was previously shown to associate with “crypts” thought to represent sites of granule release in zebrafish eggs (Becker and Hart, 1999). Furthermore, studies of fixed pancreatic acinar cells, using probes for both F-actin and exocytosing zymogen granules, have shown clearly that the remnant secretory granule membranes in these cells are likewise associated with F-actin, whereas those granules that have not undergone exocytosis are not (Nemoto et al., 2004; Turvey and Thorn, 2004). These studies were presaged by previous work on fixed samples of acinar cells showing that a subset of secretory vesicles were associated with F-actin, although it was not clear whether F-actin coating preceded or followed exocytosis (Valentijn et al., 2000). Similarly, a recent study of fixed alveolar cells has revealed that a subset of secretory vesicles are associated with F-actin (van Weeren et al., 2004), suggesting that F-actin coating is a conserved feature of exocytosis in this cell type as well. The importance of F-actin coats for the stability of the vesicle remnants is revealed by the fact that disruption of F-actin results in collapse (full fusion) of cortical granule membranes into the PM after exocytosis in Xenopus eggs (Sokac et al., 2003) and abnormal expansion of fused zymogen granules into structures that mimic those seen in pancreatitis in pancreatic acinar cells (Valentijn et al., 1999; Nemoto et al., 2004; but see also Bi and Williams, 2005).
Figure 2.
Fluorescence micrographs showing relationship between F-actin and exocytosing cortical granules in Xenopus eggs. Eggs were induced to undergo exocytosis in the presence of extracellular fluorescent dextran. (A) In a Z-section from an egg fixed and then stained for F-actin with fluorescent phalloidin (green), the dextran (red) only incorporates into exocytosing cortical granules. The panels show three different cortical granules, each in a different stage of exocytosis. Granule 1 seems to have just fused, as revealed by the presence of a small amount of dextran fixed in the opening fusion pore. Granule 2 has been fused with the PM for a longer time, as judged by partial dextran filling of the ghost and the beginning of F-actin accumulation near the pore. Granule 3 has been fused with the PM the longest, because it has the most dextran and an F-actin coat that almost completely encircles it (arrowheads) (also see Sokac et al., 2003; Figure 1). (B) In an en face view of a living egg that was previously loaded with fluorescent monomeric actin (green), dextran (red) marks exocytosing cortical granules (arrowheads). Cortical granules that have not yet fused with the PM show up as dark holes (arrows). Note that exocytosing cortical granules are surrounded by F-actin coats, whereas immediately adjacent granules that have not yet undergone exocytosis lack F-actin coats (also see Sokac et al., 2003; Figure 1).
One of the most fascinating results from the above-mentioned studies is that F-actin does not merely play a passive role during exocytosis, with stable, preexisting cortical F-actin alone being responsible for all of the sequelae of regulated exocytosis. Rather, in both Xenopus eggs and pancreatic acini, actin rapidly assembles around secretory vesicles upon exocytosis (Sokac et al., 2003; Nemoto et al., 2004). In Xenopus eggs, actin assembly starts at the fusion pore and then spreads along the entire granule surface, to actually compress the exocytosing cortical granule (Figure 2; Sokac et al., 2003). In pancreatic acini, assembling actin does not immediately compress the compartments but instead progressively encloses zymogen granules undergoing sequential exocytosis (Nemoto et al., 2004). Thus, depending on the requirements of the cell type in question, actin assembly is subjected to extraordinarily tight spatial and temporal control.
The coating of exocytosing vesicles with F-actin not only stabilizes the secretory compartment during prolonged union with the PM but also may be responsible for eventual compensatory endocytosis. In Xenopus eggs, the actin coat compresses the cortical granule membrane, at least some of which seems to be retrieved directly (Sokac et al., 2003), whereas zymogen granule membranes undergo similar compression in acinar cells after exocytosis and seem to be retrieved at least in part without collapse into the PM (Nemoto et al., 2004; Thorn et al., 2004). Furthermore, that actin coats in Xenopus eggs actually compress exocytosing granules (Sokac et al., 2003) raises the possibility that coating could also promote expulsion of secretory granule contents into the extracellular medium. These findings reveal a new and important function for F-actin in membrane trafficking in addition to its previously demonstrated roles in receptor-mediated endocytosis (Engqvist-Goldstein and Drubin, 2003; Merrifield, 2004) and modulation of membrane fusion (Vitale et al., 2001, Ehre et al., 2005).
COMPARTMENT MIXING AS A STIMULUS FOR LOCAL ACTIN ASSEMBLY
These observations prompt an obvious question: how is actin assembly limited in space and time during regulated exocytosis? At least two mechanisms could account for this specificity. Either the stimulus for exocytosis, elevated intracellular free calcium, could promote local actin assembly directly, or the fusion event itself could promote local actin assembly. Based on the following considerations, we suggest that fusion itself, and, in particular, mixing components of the secretory vesicle membrane with the PM, acts as the trigger for local actin assembly. First, analysis of actin assembly within a discrete region of the cortex of Xenopus eggs shows that actin assembly occurs specifically on cortical granules that have fused with the PM, but not their immediate unfused neighbors (Figure 2; Sokac et al., 2003). Similarly, actin coats in pancreatic acini are likewise restricted to zymogen granules that have exocytosed (Nemoto et al., 2004; Turvey and Thorn, 2004). Second, comparison of the timing of calcium elevation in Xenopus eggs versus exocytosis and actin coat assembly reveals that actin coat assembly is tightly correlated with exocytosis itself but not directly correlated with calcium elevation (Figure 3). Third, in pancreatic acini, actin assembly during sequential exocytosis is likewise directly correlated with fusion of adjacent zymogen granules, and not directly correlated with the stimulus for exocytosis itself, except insofar as the stimulus is required to initiate exocytosis (Nemoto et al., 2004).
Figure 3.
Quantification demonstrating that the timing of actin coat assembly is tightly coupled to exocytosis but not to application of the exocytotic stimulus in Xenopus eggs. To stimulate cortical granule exocytosis in living eggs bathed in extracellular fluorescent dextran and pre-loaded with labeled actin, calcium was elevated by photolysis of caged inositol trisphosphate at time 0 on the x-axis. Cortical granules underwent exocytosis at different times after uncaging (as judged by first time frame when dextran filling occurred; this filling was essentially complete within 2 s), with most granules fusing with the PM between 6 and 10.5 s after uncaging, but some fusing as late as 80 s after uncaging. The time to actin coating after uncaging was also scored for all exocytosing granules (as judged by first time frame when a complete ring of F-actin encircled an exocytosing granule). To better understand how the timing of uncaging and exocytosis relates to actin coat assembly, the time elapsed between dextran filling and actin coat assembly was calculated for individual exocytosing granules. Compare early granules that exocytosed within 28.5 s of uncaging versus late granules that exocytosed 30–80.5 s after uncaging. For both early and late exocytosing granules, there was no significant difference between the timing of dextran filling and actin coat assembly (early average of 16.3 s versus. late average of 16.1 s). This suggests that fusion of the granules with the PM rather than uncaging is the key signal for actin coating (data collected from en face, single-plane confocal movies originally used for Sokac et al., 2003). For early statistics and detailed methods, see Sokac et al., 2003.
Thus, there is a tight spatial and temporal correlation between actin assembly on exocytosing secretory vesicles and the fusion event itself, and only an indirect correlation with the stimulus for secretion, implying that fusion itself is the trigger. The two major changes that result from secretory vesicle–PM fusion are exposure of the interior of the secretory compartments to the extracellular medium and mixing of components of the PM with the components of the secretory vesicle membrane. However, actin coats form around exocytosing cortical granules in Xenopus eggs regardless of the makeup of the external medium (our unpublished data). We therefore propose that compartment mixing acts as the proximal trigger for actin assembly on exocytosing secretory vesicles, such that the union of previously separated components of the PM and secretory vesicle membranes results in rapid actin filament nucleation (Figure 4).
Figure 4.
Schematic representation of the “compartment-mixing” hypothesis. After fusion of the secretory vesicle (blue) with the PM (orange), the components of the two compartments mix and in so doing stimulate actin assembly. Consequently, F-actin (green) begins to assemble near the fusion pore and then spreads downward over the vesicle in the wake of the intermixing components of the two compartments until it completely encloses the vesicle membrane.
A compartment mixing-dependent mechanism for actin assembly has at least one obvious advantage over a mechanism triggered by calcium elevation per se. That is, F-actin is well known to suppress exocytosis by acting as a barrier to secretory vesicle–PM fusion (Ehre et al., 2005). Thus, if actin assembly were triggered as a result of calcium elevation, any secretory vesicles that failed to immediately fuse with the PM in response to calcium would nonetheless become coated with F-actin and likely be inhibited from fusing later. Given that in eggs (Terasaki, 1995; Bement et al., 2000), pancreatic acini (Nemoto et al., 2001), and aveolar type II cells (Haller et al., 1998) not all secretory vesicles fire immediately upon calcium elevation, an actin assembly mechanism directly entrained to calcium elevation would be expected to significantly reduce the number, extent, or both of functional exocytotic events.
MECHANISMS OF COMPARTMENT MIXING-DEPENDENT ACTIN ASSEMBLY
At least two nonexclusive mechanisms could result in compartment mixing-dependent actin assembly: 1) The secretory vesicle and PM each have components that when separated are insufficient to promote actin assembly, but when combined, work synergistically to drive actin filament formation. 2) Rapidly diffusing PM components are capable of directing actin assembly by themselves but are masked by slowly diffusing components in the PM, and upon secretory vesicle fusion to the PM, rapidly partition into the secretory vesicle compartment and so escape suppression.
If these mechanisms are to work as a signal for local actin assembly, the diffusional mobility of proteins, lipids, or both into or out of the membrane remnants of the secretory vesicle would have to be somewhat, but not completely, limited. Otherwise, any necessary gradients of membrane components would either be rapidly equalized upon fusion (Allersma et al., 2004) or would never develop in the first place. Although this point has not been investigated thoroughly in the examples of kiss-and-coat exocytosis cited above, analysis of fixed samples has shown that, after exocytosis, syntaxin-2, a PM SNARE, localizes to zymogen granule membrane remnants in pancreatic acinar cells (Pickett et al., 2005). Surprisingly, it has also been shown that diffusion of a lipid marker from fused secretory granule membranes into the PM is relatively minimal in the same system (Thorn et al., 2004). As suggested by the authors, this might reflect either differential mobility of particular players or differences in experimental conditions between the two studies. In Xenopus eggs, phosphatidylinositol 4,5-bisphosphate (PIP2) from the PM incorporates into exocytosing cortical granule membranes (Figure 5), as do biotinylated cell surface proteins (our unpublished data), at levels that are reduced relative to those found in the PM, consistent with the generation of local gradients within the plane of the secretory vesicle membrane.
Figure 5.
Fluorescence micrographs showing the distribution of F-actin and PIP2 in cortical granules relative to the timing of exocytosis in Xenopus eggs. Eggs expressing a PIP2 probe (the green fluorescent protein-tagged pleckstrin homology domain of phospholipase Cδ) were fixed at times before and after calcium elevation and then stained for F-actin with fluorescent phalloidin. In Z-sections, PIP2 (green) is found at the PM before elevation of calcium (PRIOR) but is not evident in cortical granules. Shortly after calcium elevation (EARLY), PIP2 spreads part way into the exocytosing cortical granule membrane (arrowhead) before any sign of F-actin coating (red). At later stages (LATE), the PIP2 is distributed throughout the cortical granule membrane and is surrounded by an F-actin coat (arrows). (For imaging methods, see Sokac et al., 2003.)
The identity of potential signals produced by compartment mixing is unknown, but at least two PM lipids are promising candidates—PIP2 and diacylglcerol (DAG). PIP2 promotes actin assembly via the N-WASP–Arp2/3 pathway both directly and indirectly (Insall and Machesky, 2004) and has been suggested as a general mediator of actin assembly during exocytosis (Cremona and De Camilli, 2001). As described above, PIP2 incorporates into exocytosing Xenopus egg cortical granule membranes. Additionally, the DAG mimic phorbol 12-myristate 13-acetate promotes actin assembly on vesicles in Xenopus eggs and egg extracts in a manner that may be N-WASP and Cdc42 dependent (Taunton et al., 2000). Because actin coat assembly in Xenopus is associated with both local Cdc42 activation and N-WASP recruitment (Sokac et al., 2003), either or both of these lipids could promote local actin assembly after incorporation into cortical granule membranes.
Regarding the molecular players likely to couple signals generated by compartment mixing to actin assembly, we are on firmer ground. The rho class GTPases, which promote actin filament assembly via various effectors, have been implicated in secretion in several systems (Bader et al., 2004) and at least two of these are required for actin coat assembly during kiss-and-coat exocytosis—Cdc42 and Rho. In Xenopus eggs, active Cdc42 localizes to exocytosing cortical granules as does N-WASP (Sokac et al., 2003), a Cdc42 target that promotes actin assembly. Furthermore, perturbing Cdc42 function prevents actin coat assembly and mimics the phenotype produced by actin disruption. In pancreatic acinar cells, disruption of Rho function likewise prevents actin coating of zymogen granules and mimics pharmacological inhibition of actin assembly (Nemoto et al., 2004), and induction of exocytosis is associated with activation of both Rho and Rac (Bi and Williams, 2005).
Dynamin represents another potential downstream target of compartment mixing-generated signals, based on several previous observations. Specifically, dynamin has been implicated in retrieval of exocytotic vesicles after kiss-and-run exocytosis (Graham et al., 2002; Holroyd et al., 2002; Tsuboi et al., 2004) and is known to promote vesicle-associated actin assembly (Lee and De Camilli, 2002; Merrifield et al., 2002; Orth et al., 2002; Schafer et al., 2002). Although the secretory compartment remnants associated with kiss-and-coat exocytosis are far larger than those typically associated with dynamin-dependent retrieval, it could potentially function by helping carve out small portions of the remnant membrane, similar to the late stages of phagocytosis (Aggeler and Werb, 1982).
COMPARTMENT MIXING-DEPENDENT ACTIN ASSEMBLY IN OTHER CONTEXTS
At present, the evidence for compartment mixing-dependent modulation of the actin cytoskeleton stems predominantly from the studies on kiss-and-coat discussed above. However, other basic cellular processes accompanied by focused actin assembly represent strong candidates to be controlled by compartment mixing—phagocytosis, cell locomotion, PM repair (cellular wound healing), cell–cell adhesion formation, and cytokinesis. During phagocytosis, actin assembles around forming phagosomes (Swanson and Hoppe, 2004). Analogous to the results from Xenopus eggs and pancreatic acini, recent studies show that rho class GTPases are progressively recruited to forming phagosomes (Hoppe and Swanson, 2004). The means by which the GTPases are activated has yet to be determined, but it has been shown that phagosome formation is accompanied by focal exocytosis (Bajno et al., 2000). Cell locomotion is accompanied by activation of rho GTPases at the leading edge of crawling cells (Kraynov et al., 2000) and membrane inserts in the same region (Schmoranzer et al., 2003). Similarly, PM damage is accompanied by both local exocytosis (Bi et al., 1995), and activation of rho class GTPases (Benink and Bement, 2005). Newly forming, E-cadherin-mediated cell–cell adhesion sites recruit exocyst components from the cytoplasm to direct local exocytosis (Grindstaff et al., 1998), and cadherin engagement activates Rho-family GTPases (Braga and Yap, 2005). Last, during cytokinesis, the furrow region has long been known as a site of local membrane insertion (Danilchik et al., 2003), and has recently been shown to be a site of local RhoA activation (Bement et al., 2005).
Although the notion that compartment mixing directs local rho GTPase activation and actin remodeling in situations beyond kiss-and-coat exocytosis has yet to be tested, this idea is particularly attractive for at least two reasons. First, in all of the above-mentioned examples, actin assembly is confined in a very precise manner at or near the PM, with immediate proximity to the site of exocytosis. Second, microtubules are required for many of the above-mentioned processes, and one of the major functions of microtubules is to direct membrane insertion (Goodson et al., 1997).
FUTURE DIRECTIONS
The analysis presented here suggests several key directions for questions to be addressed by future studies. First, in other systems of sequential exocytosis, such as cells of the immune system, is actin coating also observed around exocytosing granules? Second, what are the protein and/or lipid cues that direct actin coating? Third, how is compartment mixing coordinated with actin coating such that F-actin does not prevent secondary and tertiary fusion events? Fourth, does compartment mixing indeed trigger actin assembly in situations other than kiss-and-coat exocytosis?
Acknowledgments
We thank Tom Martin, Ed Chapman, Meyer Jackson, Guy Groblewski (University of Wisconsin, Madison), and Cameron Gunderson (UCLA, Los Angeles, CA) for sharing freely expertise on membrane fusion; Thomas Haller (University of Innsbruck, Innsbruck, Austria) and Peter Thorn (University of Cambridge, Cambridge, United Kingdom) for e-mail discussion of results; and Elsie Yu (University of Wisconsin, Madison) for critical reading of this manuscript. This study was supported by National Institutes of Health Grant GM-52932.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-10-0908) on January 25, 2006.
References
- Aggeler, J., and Werb, Z. (1982). Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane-particle interactions and clathrin. J. Cell Biol. 94, 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albillos, A., Dernick, G., Horstmann, H., Almers, W., Alvarez de Toledo, G., and Lindau, M. (1997). The exocytotic event in chromaffin cells revealed by patch amperometry. Nature 389, 509–512. [DOI] [PubMed] [Google Scholar]
- Allersma, M. W., Wang, L., Axelrod, D., and Holz, R. W. (2004). Visualization of regulated exocytosis with a granule-membrane probe using total internal reflection microscopy. Mol. Biol. Cell 15, 4658–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo, C. R., Henley, J. R., McNiven, M. A., and Palfrey, H. C. (1995). Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc. Natl. Acad. Sci. USA 92, 8328–8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo, C. R., Elhamdani, A., and Palfrey, H. C. (1998). Secretion: dense-core vesicles can kiss-and-run too. Curr. Biol. 8, R62–R65. [DOI] [PubMed] [Google Scholar]
- Bader, M. F., Doussau, F., Chasserot-Golaz, S., Vitale, N., and Gasman, S. (2004). Coupling actin and membrane dynamics during calcium-regulated exocytosis: a role for Rho and ARF GTPases. Biochim. Biophys. Acta 1742, 37–49. [DOI] [PubMed] [Google Scholar]
- Bajno, L., Peng, X. R., Schreiber, A. D., Moore, H. P., Trimble, W. S., and Grinstein, S. (2000). Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J. Cell Biol. 149, 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, K. A., and Hart, N. H. (1999). Reorganization of filamentous actin and myosin-II in zebrafish eggs correlates temporally and spatially with cortical granule exocytosis. J. Cell Sci. 112, 97–110. [DOI] [PubMed] [Google Scholar]
- Bement, W. M., Benink, H. A., Mandato, C. A., and Swelstad, B. B. (2000). Evidence for direct membrane retrieval following cortical granule exocytosis in Xenopus oocytes and eggs. J. Exp. Zool. 286, 767–775. [DOI] [PubMed] [Google Scholar]
- Bement, W. M., Benink, H. A., and von Dassow, G. (2005). A microtubule-dependent zone of active RhoA during cleavage plane specification. J. Cell Biol. 170, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benink, H. A., and Bement, W. M. (2005). Concentric zones of active RhoA and Cdc42 around single cell wounds. J. Cell Biol. 168, 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, G. Q., Alderton, J. M., and Steinhardt, R. A. (1995). Calcium-regulated exocytosis is required for cell membrane resealing. J. Cell Biol. 131, 1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, Y., and Williams, J. A. (2005). A role for Rho and Rac in secretagogue-induced amylase release by pancreatic acini. Am. J. Physiol. 289, C22–C32. [DOI] [PubMed] [Google Scholar]
- Boyle, J. A., Chen, H., and Bamburg, J. R. (2001). Sperm incorporation in Xenopus laevis: characterisation of morphological events and the role of microfilaments. Zygote 9, 167–181. [DOI] [PubMed] [Google Scholar]
- Braga, V. M., and Yap, A. S. (2005). The challenges of abundance: epithelial junctions and small GTPase signalling. Curr. Opin. Cell Biol. 17, 466–474. [DOI] [PubMed] [Google Scholar]
- Burgoyne, R. D., and Barclay, J. W. (2002). Splitting the quantum: regulation of quantal release during vesicle fusion. Trends Neurosci. 25, 176–178. [DOI] [PubMed] [Google Scholar]
- Campanella, C., and Andreuccetti, P. (1977). Ultrastructural observations on cortical endoplasmic reticulum and on residual cortical granules in the egg of Xenopus laevis. Dev. Biol. 56, 1–10. [DOI] [PubMed] [Google Scholar]
- Chen, X., Wang, L., Zhou, Y., Zheng, L. H., and Zhou, Z. (2005a). “Kiss-and-run” glutamate secretion in cultured and freshly isolated rat hippocampal astrocytes. J. Neurosci. 25, 9236–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Warner, J. D., Yule, D. I., and Giovannucci, D. R. (2005b). Spatiotemporal analysis of exocytosis in mouse parotid acinar cells. Am. J. Physiol. 289, C1209–C1219. [DOI] [PubMed] [Google Scholar]
- Cremona, O., and De Camilli, P. (2001). Phosphoinositides in membrane traffic at the synapse. J. Cell Sci. 114, 1041–1052. [DOI] [PubMed] [Google Scholar]
- Danilchik, M. V., Bedrick, S. D., Brown, E. E., and Ray, K. (2003). Furrow microtubules and localized exocytosis in cleaving Xenopus laevis embryos. J. Cell Sci. 116, 273–283. [DOI] [PubMed] [Google Scholar]
- Dietl, P., and Haller, T. (2005). Exocytosis of lung surfactant: from the secretory vesicle to the air-liquid interface. Annu. Rev. Physiol. 67, 595–621. [DOI] [PubMed] [Google Scholar]
- Ehre, C., Rossi, A. H., Abdullah, L. H., De Pestel, K., Hill, S., Olsen, J. C., and Davis, C. W. (2005). Barrier role of actin filaments in regulated mucin secretion from airway goblet cells. Am. J. Physiol. 288, C46–C56. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein, A. E., and Drubin, D. G. (2003). Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 19, 287–332. [DOI] [PubMed] [Google Scholar]
- Fesce, R., Grohovaz, F., Valtorta, F., and Meldolesi, J. (1994). Neurotransmitter release: fusion or “kiss and run”? Trends Cell Biol. 4, 1–4. [DOI] [PubMed] [Google Scholar]
- Gandhi, S. P., and Stevens, C. F. (2003). Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature 423, 607–613. [DOI] [PubMed] [Google Scholar]
- Goodson, H. V., Valetti, C., and Kreis, T. E. (1997). Motors and membrane traffic. Curr. Opin. Cell Biol. 9, 18–28. [DOI] [PubMed] [Google Scholar]
- Graham, M. E., O'Callaghan, D. W., McMahon, H. T., and Burgoyne, R. D. (2002). Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc. Natl. Acad. Sci. USA 99, 7124–7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff, K. K., Yeaman, C., Anandasabapathy, N., Hsu, S. C., Rodriguez-Boulan, E., Scheller, R. H., and Nelson, W. J. (1998). Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93, 731–740. [DOI] [PubMed] [Google Scholar]
- Haller, T., Dietl, P., Pfaller, K., Frick, M., Mair, N., Paulmichl, M., Hess, M. W., Furst, J., and Maly, K. (2001). Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J. Cell Biol. 155, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller, T., Ortmayr, J., Friedrich, F., Volkl, H., and Dietl, P. (1998). Dynamics of surfactant release in alveolar type II cells. Proc. Natl. Acad. Sci. USA 95, 1579–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel, A. W., Kang, G., and Kornhuber, J. (2001). A common molecular machinery for exocytosis and the `kiss-and-run' mechanism in chromaffin cells is controlled by phosphorylation. J. Cell Sci. 114, 4613–4620. [DOI] [PubMed] [Google Scholar]
- Heuser, J. E., and Reese, T. S. (1973). Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 57, 315–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd, P., Lang, T., Wenzel, D., De Camilli, P., and Jahn, R. (2002). Imaging direct, dynamin-dependent recapture of fusing secretory granules on plasma membrane lawns from PC12 cells. Proc. Natl. Acad. Sci. USA 99, 16806–16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe, A. D., and Swanson, J. A. (2004). Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol. Biol. Cell 15, 3509–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insall, R. H., and Machesky, L. M. (2004). Regulation of WASP: PIP2 Pipped by Toca-1? Cell 118, 140–141. [DOI] [PubMed] [Google Scholar]
- Jena, B. P., Cho, S. J., Jeremic, A., Stromer, M. H., and Abu-Hamdah, R. (2003). Structure and composition of the fusion pore. Biophys. J. 84, 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer, M., Fram, E. K., Brody, A. R., and Young, S. L. (1985). Secretion of surfactant by rat alveolar type II cells: morphometric analysis and three-dimensional reconstruction. Exp. Lung Res. 9, 351–361. [DOI] [PubMed] [Google Scholar]
- Klyachko, V. A., and Jackson, M. B. (2002). Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature 418, 89–92. [DOI] [PubMed] [Google Scholar]
- Kraynov, V. S., Chamberlain, C., Bokoch, G. M., Schwartz, M. A., Slabaugh, S., and Hahn, K. M. (2000). Localized Rac activation dynamics visualized in living cells. Science 290, 333–337. [DOI] [PubMed] [Google Scholar]
- Lee, E., and De Camilli., P. (2002). Dynamin at actin tails. Proc. Natl. Acad. Sci. USA 99, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T. T., Kishimoto, T., Hatakeyama, H., Nemoto, T., Takahashi, N., and Kasai, H. (2005). Exocytosis and endocytosis of small vesicles in PC12 cells studied with TEPIQ (two-photon extracellular polar-tracer imaging-based quantification) analysis. J. Physiol. 568, 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lollike, K., Borregaard, N., and Lindau, M. (1995). The exocytotic fusion pore of small granules has a conductance similar to an ion channel. J. Cell Biol. 129, 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil, P. L., and Steinhardt, R. A. (2003). Plasma membrane disruption: repair, prevention, adaptation. Annu. Rev. Cell Dev. Biol. 19, 697–731. [DOI] [PubMed] [Google Scholar]
- Merrifield, C. J. (2004). Seeing is believing: imaging actin dynamics at single sites of endocytosis. Trends Cell Biol. 14, 352–358. [DOI] [PubMed] [Google Scholar]
- Merrifield, C. J., Feldman, M. E., Wan, L., and Almers, W. (2002). Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 4, 691–698. [DOI] [PubMed] [Google Scholar]
- Morris, J. F., and Nordmann, J. J. (1980). Membrane recapture after hormone release from nerve endings in the neural lobe of the rat pituitary gland. Neuroscience 5, 639–659. [DOI] [PubMed] [Google Scholar]
- Murthy, V. N., Schikorski, T., Stevens, C. F., and Zhu, Y. (2001). Inactivity produces increases in neurotransmitter release and synapse size. Neuron 32, 673–682. [DOI] [PubMed] [Google Scholar]
- Neher, E., and Zucker, R. S. (1993). Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron 10, 21–30. [DOI] [PubMed] [Google Scholar]
- Nemoto, T., Kojima, T., Oshima, A., Bito, H., and Kasai, H. (2004). Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J. Biol. Chem. 279, 37544–37550. [DOI] [PubMed] [Google Scholar]
- Nemoto, T., Kimura, R., Ito, K., Tachikawa, A., Miyashita, Y., Iino, M., and Kasai, H. (2001). Sequential-replenishment mechanism of exocytosis in pancreatic acini. Nat. Cell Biol. 3, 253–258. [DOI] [PubMed] [Google Scholar]
- Orth, J. D., Krueger, E. W., Cao, H., and McNiven, M. A. (2002). The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc. Natl. Acad. Sci. USA 99, 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett, J. A., Thorn, P., and Edwardson, J. M. (2005). The plasma membrane Q-SNARE syntaxin 2 enters the zymogen granule membrane during exocytosis in the pancreatic acinar cell. J. Biol. Chem. 280, 1506–1511. [DOI] [PubMed] [Google Scholar]
- Pothos, E. N., Larsen, K. E., Krantz, D. E., Liu, Y., Haycock, J. W., Setlik, W., Gershon, M. D., Edwards, R. H., and Sulzer, D. (2000). Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J. Neurosci. 20, 7297–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, A., Caler, E. V., and Andrews, N. W. (2001). Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell 106, 157–169. [DOI] [PubMed] [Google Scholar]
- Richards, D. A., Bai, J., and Chapman, E. R. (2005). Two modes of exocytosis at hippocampal synapses revealed by rate of FM1–43 efflux from individual vesicles. J. Cell Biol. 168, 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, D. A., Weed, S. A., Binns, D., Karginov, A. V., Parsons, J. T., and Cooper, J. A. (2002). Dynamin2 and cortactin regulate actin assembly and filament organization. Curr. Biol. 12, 1852–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoranzer, J., Kreitzer, G., and Simon, S. M. (2003). Migrating fibroblasts perform polarized, microtubule-dependent exocytosis towards the leading edge. J. Cell Sci. 116, 4513–4519. [DOI] [PubMed] [Google Scholar]
- Schneider, S. W. (2001). Kiss and run mechanism in exocytosis. J. Membr. Biol. 181, 67–76. [PubMed] [Google Scholar]
- Schneider, S. W., Sritharan, K. C., Geibel, J. P., Oberleithner, H., and Jena, B. P. (1997). Surface dynamics in living acinar cells imaged by atomic force microscopy: identification of plasma membrane structures involved in exocytosis. Proc. Natl. Acad. Sci. USA 94, 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokac, A. M., Co, C., Taunton, J., and Bement, W. M. (2003). Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat. Cell Biol. 5, 727–732. [DOI] [PubMed] [Google Scholar]
- Staal, R. G., Mosharov, E. V., and Sulzer, D. (2004). Dopamine neurons release transmitter via a flickering fusion pore. Nat. Neurosci. 7, 341–346. [DOI] [PubMed] [Google Scholar]
- Swanson, J. A., and Hoppe, A. D. (2004). The coordination of signaling during Fc receptor-mediated phagocytosis. J. Leukoc. Biol. 76, 1093–1103. [DOI] [PubMed] [Google Scholar]
- Taunton, J., Rowning, B. A., Coughlin, M. L., Wu, M., Moon, R. T., Mitchison, T. J., and Larabell, C. A. (2000). Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J. Cell Biol. 148, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki, M. (1995). Visualization of exocytosis during sea urchin egg fertilization using confocal microscopy. J. Cell Sci. 108, 2293–2300. [DOI] [PubMed] [Google Scholar]
- Thorn, P., Fogarty, K. E., and Parker, I. (2004). Zymogen granule exocytosis is characterized by long fusion pore openings and preservation of vesicle lipid identity. Proc. Natl. Acad. Sci. USA 101, 6774–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn, P., and Parker, I. (2005). Two phases of zymogen granule lifetime in mouse pancreas: ghost granules linger after exocytosis of contents. J. Physiol. 563, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi, T., McMahon, H. T., and Rutter, G. A. (2004). Mechanisms of dense core vesicle recapture following “kiss and run” (“cavicapture”) exocytosis in insulin-secreting cells. J. Biol. Chem. 279, 47115–47124. [DOI] [PubMed] [Google Scholar]
- Turvey, M. R., and Thorn, P. (2004). Lysine-fixable dye tracing of exocytosis shows F-actin coating is a step that follows granule fusion in pancreatic acinar cells. Pflueg. Arch. Eur. J. Physiol. 448, 552–555. [DOI] [PubMed] [Google Scholar]
- Valentijn, J. A., Valentijn, K., Pastore, L. M., and Jamieson, J. D. (2000). Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc. Natl. Acad. Sci. USA 97, 1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn, K. M., Gumkowski, F. D., and Jamieson, J. D. (1999). The subapical actin cytoskeleton regulates secretion and membrane retrieval in pancreatic acinar cells. J. Cell Sci. 112, 81–96. [DOI] [PubMed] [Google Scholar]
- Valtorta, F., Meldolesi, J., and Fesce, R. (2001). Synaptic vesicles: is kissing a matter of competence? Trends Cell Biol. 11, 324–328. [DOI] [PubMed] [Google Scholar]
- van Weeren, L., de Graaff, A. M., Jamieson, J. D., Batenburg, J. J., and Valentijn, J. A. (2004). Rab3D and actin reveal distinct lamellar body subpopulations in alveolar epithelial type II cells. Am. J. Respir. Cell Mol. Biol. 30, 288–295. [DOI] [PubMed] [Google Scholar]
- Vitale, N., Caumont, A. S., Chasserot-Golaz, S., Du, G., Wu, S., Sciorra, V. A., Morris, A. J., Frohman, M. A., and Bader, M. F. (2001). Phospholipase D 1, a key factor for the exocytotic machinery in neuroendocrine cells. EMBO J. 20, 2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. T., Lu, J. C., Bai, J., Chang, P. Y., Martin, T. F., Chapman, E. R., and Jackson, M. B. (2003). Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature 424, 943–947. [DOI] [PubMed] [Google Scholar]
- Wessel, G. M., Brooks, J. M., Green, E., Haley, S., Voronina, E., Wong, J., Zaydfudim, V., and Conner, S. (2001). The biology of cortical granules. Int. Rev. Cytol. 209, 117–206. [DOI] [PubMed] [Google Scholar]
- Whalley, T., Terasaki, M., Cho, M. S., and Vogel, S. S. (1995). Direct membrane retrieval into large vesicles after exocytosis in sea urchin eggs. J. Cell Biol. 131, 1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman, R. M., and Haynes, C. L. (2004). Synaptic vesicles really do kiss and run. Nat. Neurosci. 7, 321–322. [DOI] [PubMed] [Google Scholar]