Abstract
Superoxide damages dehydratases that contain catalytic [4Fe-4S]2+ clusters. Aconitases are members of that enzyme family, and previous work showed that most aconitase activity is lost when Escherichia coli is exposed to superoxide stress. More recently it was determined that E. coli synthesizes at least two isozymes of aconitase, AcnA and AcnB. Synthesis of AcnA, the less-abundant enzyme, is positively controlled by SoxS, a protein that is activated in the presence of superoxide-generating chemicals. We have determined that this arrangement exists because AcnA is resistant to superoxide in vivo. Surprisingly, purified AcnA is extremely sensitive to superoxide and other chemical oxidants unless it is combined with an uncharacterized factor that is present in cell extracts. In contrast, AcnB is highly sensitive to a variety of chemical oxidants in vivo, in extracts, and in its purified form. Thus, the induction of AcnA during oxidative stress provides a mechanism to circumvent a block in the tricarboxylic acid cycle. AcnA appears to be as catalytically competent as AcnB, so the retention of the latter as the primary housekeeping enzyme must provide some other advantage. We observed that the [4Fe-4S] cluster of AcnB is in dynamic equilibrium with the surrounding iron pool, so that AcnB is rapidly demetallated when intracellular iron pools drop. AcnA and other dehydratases do not show this trait. Demetallated AcnB is known to bind its cognate mRNA. The absence of AcnB activity also causes the accumulation and excretion of citrate, an iron chelator for which E. coli synthesizes a transport system. Thus, AcnB may be retained as the primary aconitase because the lability of its exposed cluster allows E. coli to sense and respond to iron depletion.
Superoxide is generated inside aerobic organisms when molecular oxygen adventitiously oxidizes the cofactors of redox enzymes (27, 41). The rate of O2− formation is sufficiently high that superoxide inhibits the growth of organisms that cannot scavenge it. For example, mutants of Escherichia coli that lack cytosolic superoxide dismutase (SOD) cannot grow in air unless a fermentable carbon source and branched-chain, aromatic, and sulfurous amino acids are all provided in the medium (5). Several of these phenotypes result from the ability of superoxide to inactivate members of a family of dehydratases that utilize [4Fe-4S] clusters as catalytic Lewis acids (14). Superoxide is electrostatically attracted to the exposed [4Fe-4S]2+ clusters and efficiently (k = 106 to 107 M−1 s−1) oxidizes them to an unstable [4Fe-4S]3+ state. The oxidized cluster spontaneously degrades to the [3Fe-4S]1+ form, with loss of the catalytic iron atom to the bulk solution. The consequence is that the demetallated enzyme is inactive and the pathway to which it belongs is disabled.
Dihydroxyacid dehydratase, fumarases A and B, and 6-phosphogluconate dehydratase are among the well-characterized enzymes in E. coli that are vulnerable to this injury (13, 17, 34, 35). Damage to dihydroxyacid dehydratase causes SOD mutants to be unable to synthesize branched-chain amino acids, while the inactivity of fumarase presumably prohibits growth on nonfermentable carbon sources, which require a large tricarboxylic acid (TCA) cycle flux for energy production. A secondary effect of the cluster damage is that the released iron spills into the cytosol, where it can react with hydrogen peroxide and cause DNA damage (30, 31, 36). Thus, SOD mutants have a high rate of spontaneous mutation when they are grown in air (10).
E. coli responds to superoxide stress by activating the SoxRS regulon (23, 48). SoxS protein stimulates the synthesis of about a dozen proteins (45), including SOD (25), which reduces the steady-state level of superoxide, and endonuclease IV (6), a DNA repair enzyme that corrects oxidative lesions. One other SoxRS-regulated enzyme is fumarase C (35). Fumarase C is an isozyme of fumarases A and B, but unlike them FumC has no iron-sulfur cluster and is fully resistant to superoxide. Thus, its induction should permit some flux through the TCA cycle when fumarases A and B are damaged by superoxide.
However, prior work has shown that aconitase is also very sensitive to superoxide (18, 26). If this TCA cycle enzyme were inactivated by superoxide, the induction of fumarase C would seemingly be futile. A clue to this puzzle was the discovery that E. coli actually contains two aconitase enzymes (24). Both process substrate, as an auxotrophy for 2-oxoglutarate is only observed when both structural genes are mutated. Aconitase B possesses the greater activity in crude cell extracts, while aconitase A is induced upon activation of SoxRS.
The parallel between the aconitase isozymes and the fumarase isozymes suggested the hypothesis that aconitase A, like fumarase C, might be a superoxide-resistant enzyme whose induction compensates for damage to the housekeeping isozyme. The difficulty with this idea, however, is that the primary amino acid sequence of aconitase A indicated that it possesses a [4Fe-4S] cluster. Electron paramagnetic resonance studies of the purified enzyme confirmed that this is so (3). Because other [4Fe-4S] dehydratases are oxidant sensitive, it seemed peculiar that SoxRS would induce AcnA during periods of oxidative stress.
AcnA is also positively regulated by Fur, the global iron regulatory protein; AcnB is not (24). Thus, an alternative explanation for the presence of two cluster-containing aconitases might be that one or both play a role in iron metabolism. In mammalian cells, mitochondrial aconitase drives the TCA cycle while a cytosolic “aconitase” isozyme, denoted IRP (iron regulatory protein), serves as an intracellular iron sensor (29, 33). The solvent-exposed iron atom of its cluster reversibly dissociates from it; thus, when cellular iron pools are low, the cluster is demetallated. The apoprotein then binds mRNAs that encode enzymes critical for iron metabolism, stabilizing the messages of iron receptors while destabilizing those of iron-storage proteins. Tang and Guest have shown that the purified, apoenzyme forms of AcnA and AcnB from E. coli both specifically bind their cognate mRNAs (47). How these activities might be integrated into control of iron metabolism is not yet clear.
The purpose of this study was to characterize the stabilities of these enzymes. The data indicate that both of these enzymes differ from most [4Fe-4S] dehydratases. Under normal conditions, AcnB processes the bulk of the metabolic flux; it is sensitive to both oxidative stress and, independently, iron depletion. In contrast, AcnA is resistant in vivo both to oxidative stress and to iron withdrawal.
MATERIALS AND METHODS
Media and growth curves.
Most chemicals were purchased from Sigma. Sodium citrate was from EM Science; ether 650S was from Tosohaas; oxidized glutathione was from Boehringer Mannheim; isopropyl-β-d-thiogalactoside (IPTG) was from Research Products International Corp.; beef liver catalase was from Roche; and d,l-lactic acid and MnCl2 were from Aldrich.
Luria-Bertani (LB) broth consisted of 10 g of Bacto-tryptone, 5 g of yeast extract, and 10 g of NaCl per liter (42) and was supplemented as required with tetracycline (12 μg/ml), kanamycin (30 μg/ml), chloramphenicol (20 μg/ml), and ampicillin (200 μg/ml). Defined media contained minimal A salts (42) and either 0.2% glucose, 0.2% gluconate, or 50 mM lactate. Either 0.2% Casamino Acids or a 0.5 mM concentration of the desired amino acids was added. To measure the rate of exponential growth, cells from an overnight culture were diluted to an optical density at 600 nm (OD600) of 0.010, cultured to an OD600 of 0.100, diluted again to an OD of 0.010, and then monitored by absorbance at 600 nm. In some experiments, sufficient paraquat (20 μM to 2 mM, depending upon the medium) was added to create intracellular superoxide stress and noticeably diminish the growth of wild-type cultures.
Strains of E. coli K-12.
W3110 [IN(rrnD-rrnE)1 and its isogenic derivatives JRG2789 (acnA::kan), JRG3258 (acnB::tet), and JRG3259 (acnA::kan acnB::tet)] were generously provided by John Guest, as were the pET21a-based expression plasmids pGS1203 (acnA) in JRG4004 and pGS783 (acnB) in JRG3099 (47). SMV3 [acnA::kan (sodA::Mu d PR13)25 (sodB-kan)1-Δ2] and SMV7 [acnB::tet (sodA::Mu d PR13)25 (sodB-kan)1-Δ2] were generated by P1 transduction of the sod alleles into the acn mutant strains. The sodA mutant allele was selected on chloramphenicol plates and screened by SOD activity gel (2). In the case of SMV7, the sodB mutant allele was selected on kanamycin plates; to create SMV3, the allele was first linked to zdg299::Tn10 and then transduced into the acn parent with selection for tetracycline resistance. The tetracycline marker was then excised via the fusaric acid method (40). SMV10 (acnB::tet katE katG) was generated by P1 transducing the acnB mutation into UM1 (katE katG14 lacY spsL thi-1) (37) and selecting for tetracycline resistance; SMV11 (acnA::kan katE katG) was generated by transducing acnA::kan into UM1 and selecting for kanamycin resistance.
Preparation of cell extracts.
Cells were grown overnight in aerobic LB medium for aconitase assays and anaerobic LB medium for fumarase assays. Growth was in gluconate-Casamino Acids medium for 6-phosphogluconate dehydratase assays. The cells were then subcultured into 600 ml of LB and grown until the OD600 reached 0.8 to 1.0. The culture was centrifuged, and the cell pellets were transferred into a Coy anaerobic chamber (85% N2, 10% H2, 5% CO2), where all subsequent manipulations were performed. Anaerobiosis prevented oxidative damage from occurring to proteins in the extract. The pellets were resuspended in 2.4 ml of cold, anaerobic 50 mM Tris-Cl, pH 7.6, and lysed by sonication. The lysate was clarified by centrifugation at top speed in a microcentrifuge for 30 s. The supernatant was decanted and promptly frozen in a dry ice-ethanol bath. The extracts were thawed prior to assaying.
Enzyme assays.
Aconitase was assayed by the production of cis-aconitate (3.6 mM−1 cm−1 at 240 nm). Reaction mixtures contained enzyme and either 30 mM isocitrate or 30 mM citrate in 50 mM Tris-HCl, pH 7.4. All assay components were dissolved in anaerobic water or buffer, and reaction mixtures were assembled in the anaerobic chamber. Sealed cuvettes were then moved to the spectrophotometer. Rates were determined from the first 4 min of the assay; linearity was lost when AcnB was assayed with citrate as substrate (see Results).
The two-step method of Fraenkel and Horecker was used to determine 6-phosphogluconate dehydratase activity (15). Fumarase activity was assayed by the conversion of l-malate to fumarate (32). As described for the aconitase assays, reaction mixtures were assembled anaerobically and monitored in sealed cuvettes. The fumarase activity of anaerobic cells was almost entirely derived from fumarases A and B (rather than the stable isozyme, fumarase C), since activity was lost when extracts were exposed to superoxide, as described below. Protein was measured by the Coomassie dye-binding assay (Pierce), using ovalbumin as a standard.
Citrate measurements.
Cells were grown aerobically in glucose-Casamino Acids medium. Two consecutive subcultures were performed as described above to ensure that cells were in exponential phase. When cultures reached an OD600 of 0.4 to 0.6, they were split and 20 μM paraquat was added to create superoxide stress. Unstressed cells were harvested when the cultures reached an OD600 of 0.8 to 1.0. Oxidatively stressed cells were grown for 2 h in the presence of paraquat and then harvested, also at an OD600 of 0.8 to 1.0. Cultures were centrifuged, spent media were collected and frozen, and cell pellets were washed in cold 20 mM Tris-HCl, pH 8.0. Perchloric acid (1.2 ml of 1.3 M) was added to the pellets, and the mixture was held on ice for 10 min. The perchloric acid-treated mixtures were clarified by centrifugation at top speed in a microcentrifuge for 1 min. The supernatant was collected and 0.6 ml of 0.75 M NaHCO3 was added. This mixture was placed on ice for 15 min; debris was then removed by centrifugation at 10,000 × g for 5 min. The supernatant was collected and frozen. Prior to assaying for citrate, media and supernatant were thawed, and the pH was adjusted to 7.5 to 8.0. Citrate was measured by using a kit from Boehringer Mannheim. Amounts of citrate were converted to intracellular concentrations using a conversion factor derived from the fact that 1 ml of a 1-OD culture of E. coli contains a total cytoplasmic volume of 0.5 μl (27).
Exposure of enzymes to oxidants.
To expose aconitase (purified and in extracts) to O2−, enzyme was added to aerobic buffer containing xanthine and xanthine oxidase (21). Either SOD (810 U/ml) or catalase (500 U/ml) was added to determine whether O2− or H2O2, respectively, were responsible for enzyme inactivation. Inactivation by molecular oxygen per se was determined in the absence of xanthine oxidase; SOD and catalase were added to the buffer, since oxidation of components of the cell extract can generate some superoxide and H2O2. Enzymes were exposed to H2O2, and ferricyanide addition was conducted anaerobically. In some cases the inactivated aconitase was subsequently reactivated by the addition of 5 mM dithiothreitol (DTT) and 1 mM Fe(NH4)2(SO4)2.
The effect of H2O2 upon enzymes in vivo was tested by the addition of H2O2 to catalase-deficient cells suspended in buffer. At each time point, catalase was added and the cells were centrifuged and frozen in dry ice-ethanol. The cells were later thawed, resuspended in cold 50 mM Tris-HCl (pH 7.4), and ruptured by sonication. The extract was clarified and assayed for fumarase and aconitase. Control cells were treated in the same way, except that H2O2 was not added.
The stability of H2O2 and Fe2+ in samples was confirmed by horseradish peroxidase-o-dianisidine (43) and ferene (14) assays, respectively. The ferene assay was also used to quantify the release of ferrous iron during the oxidation of AcnA. Purified AcnA was diluted into 40 mM Tris, pH 7.4, and aerated by stirring in a 3-ml cuvette. The solution also contained 1 mM ferene, 3,600 U of catalase, 200 μM xanthine, and 7 U of xanthine oxidase/ml. SOD (810 U/ml) was added to control samples. Absorbance was monitored at 562 nm.
Overproduction and purification of aconitases.
One-liter cultures of JRG4004 (overproducing aconitase A) and JRG3099 (overproducing aconitase B) were grown to an OD595 of 0.5 at 37°C. Expression of the plasmid-encoded aconitases was induced by the addition of 1 mM IPTG either for 4 h at 25°C (aconitase A) or for 2 h at 37°C (aconitase B) (47). Cultures were harvested by centrifugation at 15,000 × g for 5 min at 4°C. The pellets were then moved into the anaerobic chamber, resuspended in 20 ml of anaerobic 40 mM Tris-HCl, pH 8.0, and disrupted by sonication. The extracts were clarified by centrifugation at 8,000 rpm for 10 min. The supernatant was decanted, and 0.25 mM NH4Fe(SO4)2 and 2.5 mM DTT were added to reactivate enzyme that lacked intact iron-sulfur clusters. The extract was then loaded onto a DEAE-Sepharose column (16 mm by 35 mm), washed with five volumes of 40 mM Tris-HCl, pH 8.0, and eluted with a 300-ml linear gradient of 0 to 0.2 M sodium citrate in the same buffer. Yellowish fractions were tested for aconitase activity, and the three fractions containing the highest activity were pooled. Ammonium sulfate (1.7 M) was added, and the enzyme was loaded onto an ether 650S column (16 mm by 40 mm). The column was washed with five volumes of 40 mM Tris-HCl, pH 8.0, containing 1.7 M (NH4)2SO4. Aconitase was then eluted with a linear 200-ml gradient of 1.7 to 0 M (NH4)2SO4 in the same buffer. Fractions were assayed for aconitase activity and frozen on dry ice-ethanol. AcnA did not require reactivation upon purification, while AcnB required reactivation (28). Reactivation of purified aconitase was accomplished by the addition of 140 μM Fe(NH4)2(SO4)2, 120 μM Na2S, and 1 mM DTT for 30 min.
RESULTS
Aconitase B is unstable in vitro unless iron is supplied in the buffer.
In initial experiments with aerobic extracts of acnA and acnB mutants, aconitase activity was unstable when the sole isozyme present was AcnB and stable when the sole isozyme was AcnA (data not shown). Previous studies indicated that much of the aconitase activity of E. coli can be inactivated by superoxide that is generated spontaneously in cell extracts (18). However, when the experiments were repeated anaerobically, AcnB continued to exhibit substantial instability, even though the absence of oxygen precluded the formation of superoxide (and other oxygen species). Further study revealed that the AcnB isozyme was stable in anaerobic extracts only when it was harvested at high protein concentrations (ca. 25 mg/ml) (Fig. 1A). When extracts were generated at low protein concentrations (e.g., 0.1 mg/ml), the activity rapidly declined to a low steady-state level. Similarly, dilution (1/100) of concentrated extract caused a loss of AcnB activity, with a half-life that varied from preparation to preparation but was always <10 min.
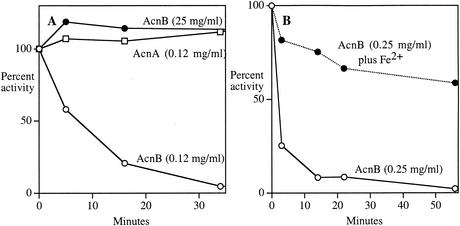
FIG. 1.
The activity of AcnB, but not AcnA, depends upon iron concentration. (A) Cell extracts containing AcnB (from JRG2789) or AcnA (from JRG3258) were suspended at the indicated protein concentrations in anaerobic 50 mM Tris-HCl and incubated at room temperature. At indicated time points, aliquots were removed and assayed under anaerobic conditions. (B) Cell extracts of JRG2789 (25 mg of protein/ml) containing AcnB were diluted into anaerobic Tris buffer. Where indicated, 1 mM Fe(NH4)2(SO4)2 was included in the buffer. Assay error was <7%; replicate experiments exhibited small variation in inactivation rate.
Virtually all activity was lost when concentrated extracts were dialyzed, suggesting that a dissociable small molecule was essential for AcnB activity.
The mammalian cytoplasmic iron-responsive element (IRE)-binding protein, which when metallated has the structure and activity of aconitase, loses catalytic activity when iron levels are low, and we therefore tested whether iron might be the molecule that stabilizes AcnB. Figure 1B shows that AcnB retained activity upon dilution when iron was provided in the diluent. Subsequent experiments showed that this stability could be achieved with concentrations of iron as low as 5 to 15 μM (data not shown). We could not infer a dissociation constant from these experiments, because ferrous iron interacts strongly with buffers and biomolecules in the extract, so that the concentration of available iron was not known.
AcnB activity also rapidly diminished when concentrated anaerobic extracts were exposed to the divalent chelator EDTA (t1/2 < 10 min) (Fig. 2A). Saturation of EDTA with ferrous iron blocked this effect. When AcnB was inactivated by either EDTA treatment or dilution, approximately 95% of the activity was restored upon subsequent treatment with iron and either reduced glutathione or DTT. Thus, it appears that the catalytic iron atom of the [4Fe-4S] cluster equilibrates between the cluster-bound and free state, so that activity is quickly lost when iron is not available in solution for reassociation with the enzyme.
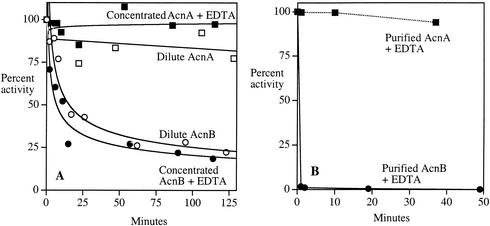
FIG. 2.
AcnB is inactivated by EDTA. (A) Extracts containing AcnB (JRG2789) (circles) or AcnA (JRG3258) (squares) were either exposed to 300 μM EDTA (25 mg of total protein/ml) (filled symbols) or diluted to 0.25 mg of total protein/ml (open symbols). Extract preparation, incubation, and assays were performed anaerobically. Neither concentrated extract lost activity when incubated in the absence of EDTA (results not shown). (B) Purified AcnA and AcnB were exposed to 300 μM EDTA anaerobically. At the indicated time points, the remaining aconitase activity was determined. Representative experiments of >3 replicates are shown, with assay errors of <10% of full-scale (100%) activity.
AcnB was purified under anaerobic conditions from a strain that overproduced it. Although initial extract activities were high, the activity quickly disappeared during purification, presumably because the local concentration of iron had fallen. It was restored after the final purification step by treatment of the purified protein with ferrous iron and either glutathione or DTT. This purified and reactivated enzyme again rapidly lost activity when EDTA was added to trap dissociated iron (Fig. 2B).
Substrates typically protect the active sites of dehydratases (18). Citrate (30 mM) substantially protected AcnB against demetallation, and isocitrate was even more effective (Fig. 3A). The results conform with the greater affinity of the active site for isocitrate (Km = 1 mM) than for citrate (Km = 11 mM) (28).
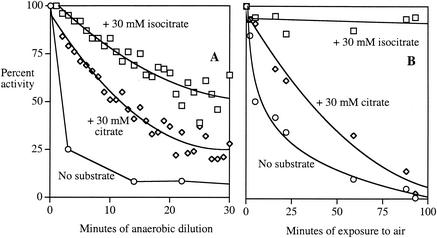
FIG. 3.
Substrates protect AcnB against iron loss and oxidation. (A) Concentrated extracts containing AcnB (JRG2789) were diluted to 0.25 mg/ml anaerobically in 50 mM Tris-HCl without or with 30 mM citrate or 30 mM isocitrate. For the latter two samples, the loss of activity was deduced from the rate of the ongoing reaction. The activity of the sample lacking substrate was determined at indicated time points by removal and assay of aliquots. Over the same period of time, concentrated enzyme lost no activity (data not shown). (B) Concentrated anaerobic extracts of SMV3 (AcnB+ AcnA− SOD−) were exposed to air with or without 30 mM citrate or 30 mM isocitrate. Aconitase activity was determined at the indicated time points. Representative experiments of >3 replicates are shown; assay variability was <10%.
Unlike AcnB, AcnA and other dehydratases do not lose activity in vitro when iron concentrations are low.
The sensitivity of AcnB activity to iron concentration is not typical of [4Fe-4S] enzymes of E. coli. For example, after 90 min of anaerobic EDTA treatment, 6-phosphogluconate dehydratase had not lost any activity, and fumarase B retained 73% of its initial activity.
Seventeen of the 20 active-site residues of AcnA and AcnB are identical, including those which coordinate the iron-sulfur cluster (24). However, in contrast to AcnB, AcnA was stable both to dilution and to treatment with EDTA (Fig. 2). During anaerobic purification AcnA maintained its activity; incubation with iron and DTT increased its activity only marginally (ca. 20%) after purification. Thus, iron atoms do not rapidly dissociate from its cluster.
AcnB is unstable under low-iron conditions in vivo.
AcnB activity was rapidly lost (t1/2 < 5 min) when dipyridyl, a cell-permeable iron chelator, was added to anaerobic acnA mutant cells (data not shown). AcnA (in the acnB mutant) was completely resistant to this treatment. This result indicates that the AcnB enzyme is in equilibrium with the intracellular iron pool in vivo.
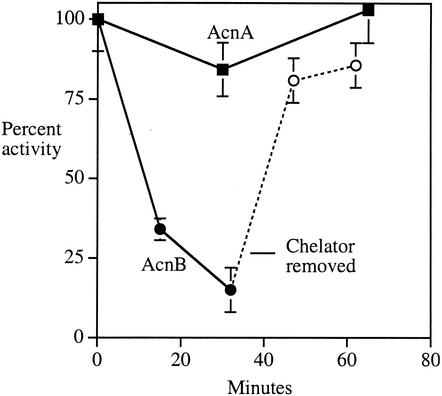
DETAPAC (diethylenetriaminepentaacetic acid), an extracellular iron chelator, was added to anaerobic cultures in order to initiate iron starvation. AcnB lost activity gradually (Fig. 4). When cells were washed free of DETAPAC and resuspended in fresh medium, the AcnB activity was rapidly restored, even when chloramphenicol was present to block new protein synthesis. Thus, iron starvation can lower the level of intracellular iron far enough to demetallate AcnB, and the inactive enzyme remains in a form that can be readily reactivated. In contrast, AcnA activity was fully resistant to DETAPAC treatment in vivo.
FIG. 4.
Aconitase B activity declines in vivo upon iron starvation. Anaerobic cultures of AcnB-containing (JRG 2789) and AcnA-containing (JRG3258) cells were exposed to 1 mM extracellular iron chelator DETAPAC during exponential growth. At the indicated time points, cells were harvested and aconitase activity assayed. At the indicated point, JRG2789 cells were pelleted, washed free of DETAPAC, and resuspended in fresh medium containing chloramphenicol (dashed line) so that intracellular enzyme repair could be observed.
AcnB is sensitive to oxidants in extracts, but AcnA is resistant.
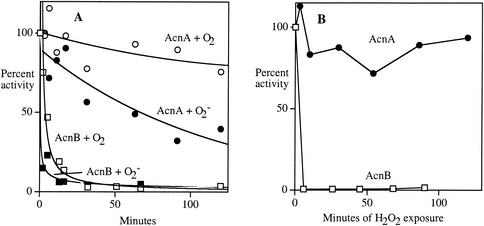
The induction of AcnA during oxidative stress (24) would make physiological sense were AcnA resistant to oxidation. The sensitivities of AcnA and AcnB to superoxide, ferricyanide, and hydrogen peroxide were initially determined in vitro using cell extracts. Concentrated extracts were prepared from anaerobic cultures of SOD− mutants that expressed only one of the two aconitases; they were then exposed to superoxide generated by xanthine oxidase in aerobic buffer. Superoxide rapidly inactivated AcnB, whereas AcnA was much more resistant to this treatment (Fig. 5A). AcnB was also rapidly inactivated when exposed to H2O2 and ferricyanide; again, AcnA was resistant (Fig. 5B and C).
FIG. 5.
Aconitase B is highly sensitive to oxidants in cell extracts, but AcnA is resistant. (A) Concentrated SOD-deficient extracts containing AcnB (SMV3) (squares) or AcnA (SMV7) (circles) were exposed to oxygen (open symbols) and superoxide (closed symbols) as described in Materials and Methods. (B) Concentrated catalase-deficient extracts containing AcnB (SMV11) (squares) or AcnA (SMV11) (circles) were exposed to 4 mM H2O2 anaerobically. (C) Concentrated extracts containing AcnB (JRG2789) (squares) and AcnA (JRG3258) (circles) were exposed to 400 μM ferricyanide. Time courses are representative of at least three experiments.
Citrate partially protected AcnB against oxidation by air, whereas saturating concentrations of isocitrate were fully protective (Fig. 3B). Similar results were observed with other oxidants (data not shown). These data match the ability of these substrates to protect against anaerobic cluster demetallation and indicate that oxidative injury occurs only when the active site cluster is accessible to the oxidant. Oxidized AcnB could be reactivated to >80% of the original activity by treatment with ferrous iron and DTT, confirming that the loss of activity was due to disruption of the catalytic [4Fe-4S] cluster. Unlike the anaerobically demetallated enzyme, oxidized AcnB could not be reactivated using glutathione in place of DTT (<10% reactivation), presumably because the oxidized enzyme requires a strong electron donor to rereduce the cluster.
Quantitative determinations of oxidant sensitivity could not be repeated with purified AcnB because upon purification AcnB required treatment with DTT and ferrous iron for reactivation. Those chemicals scavenge oxidants and repair damaged clusters, thereby confounding efforts to quantify AcnB sensitivity to oxidants. Unfortunately, the removal of DTT and iron by dialysis resulted in immediate loss of AcnB activity, due to demetallation. Qualitatively, however, exposure of the purified AcnB to oxidants did cause rapid inactivation, as expected from its behavior in extracts.
However, purified AcnA is sensitive to oxidation.
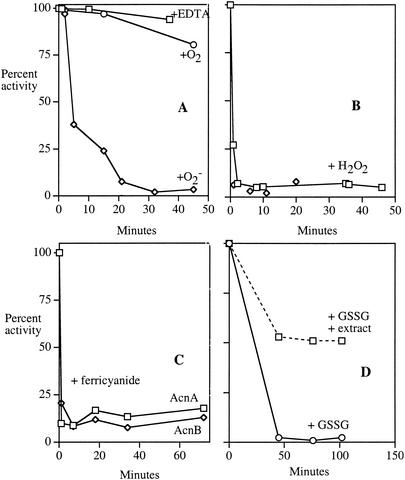
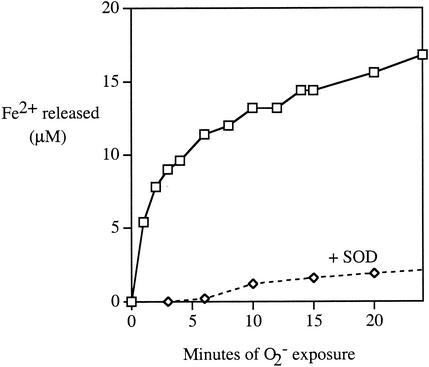
In contrast to its behavior in cell extracts, purified AcnA was extremely sensitive to superoxide, hydrogen peroxide, and ferricyanide (Fig. 6A to C). Ferrous iron was released, as occurs upon the oxidation of other dehydratase clusters (Fig. 7) (14). Unlike AcnB, however, purified AcnA was still resistant to oxygen and EDTA (Fig. 6A), indicating that its iron-sulfur cluster does not spontaneously demetallate.
FIG. 6.
When purified, AcnA remains resistant to EDTA but becomes sensitive to oxidants. Purified AcnA (7 U/ml in 40 mM Tris-HCl, pH 8.0) was exposed to 300 μM EDTA anaerobically or oxygen and superoxide in air (see Materials and Methods) (A); 400 μM (squares) or 4 mM (diamonds) H2O2 anaerobically (B); 400 μM ferricyanide anaerobically (C); or 5 mM oxidized glutathione anaerobically (D). At indicated time points, aliquots were removed and residual aconitase activity was measured. In panel D, 2.5 mg of extract/ml was added to the indicated sample. The extract itself contributed insignificantly to the aconitase activity (data not shown). Time courses are representative of at least three experiments
FIG. 7.
Ferrous iron is released when purified AcnA is inactivated by superoxide. Purified AcnA (7 μM) was exposed to the xanthine oxidase superoxide-generating system in aerobic buffer. Released Fe2+ was detected by formation of a colored complex with ferrene. Catalase was present to scavenge H2O2; where indicated, SOD was added to scavenge superoxide. Most (>80%) aconitase activity was absent by the first time point.
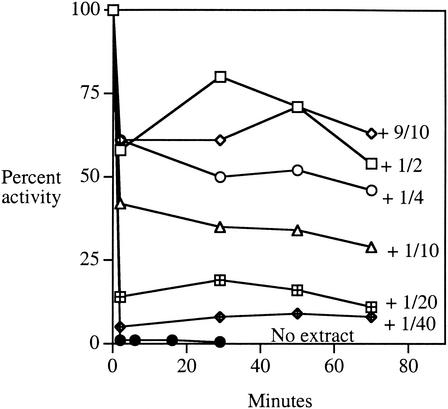
When cell extracts were added back to purified AcnA, the enzyme was once again protected from these oxidants (Fig. 8). When limiting amounts of extract were added, inactivation curves were biphasic, with the fraction of enzyme that was protected reflecting the amount of extract added. These results suggested that a titratable factor present in the extracts either protects AcnA from oxidation or rapidly reverses the damage. The factor was resistant to RNase treatment and dialysis (cutoff, 16,000 Da) but was sensitive to boiling (data not shown). The factor did not degrade H2O2, and it did not reactivate enzyme that had previously been damaged. Control proteins, such as bovine serum albumin, failed to protect AcnA. All these data suggest that extracts may contain a specific protein that adheres to AcnA. Attempts to purify the protective factor are under way.
FIG. 8.
Cell extracts contain a factor that protects purified AcnA against oxidation. Extracts of SMV10 (AcnA+ AcnB− catalase−) were generated anaerobically and added in different proportions to 7 U of purified AcnA/ml in 50 mM Tris-HCl, pH 8. After 15 min the mixtures were exposed to 4 mM H2O2. At intervals, aliquots were removed, H2O2 was scavenged with catalase, and residual aconitase activity was assayed. Aconitase activity from the extract accounted for <2% of the signal. Undiluted extract contained 25 mg of total protein/ml; the dilution factors indicate the dilution of this extract into the sample prior to H2O2 exposure. Assay variability was <10%.
AcnB is sensitive to oxidation in vivo, but AcnA is resistant.
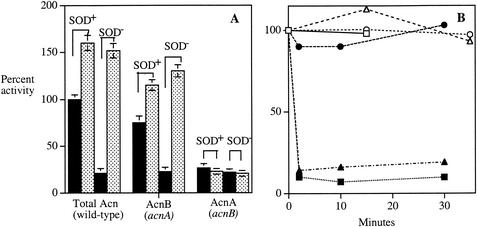
Examination of unstressed cells with either acnA or acnB mutations confirmed that about 80% of the aconitase activity of a wild-type strain is due to AcnB (Fig. 9A). A similar fraction of activity, presumably from AcnB, was lost when the wild-type extracts were incubated with EDTA (data not shown). Virtually all of the other activity was due to AcnA, since acnA acnB double mutants retained only trace (<5%) activity. (This residual activity is due to the nonspecific action of a 2-methylcitrate dehydratase [4].) Interestingly, the extracts from wild-type cells consistently gained activity when they were treated with DTT and ferrous iron, suggesting either that a substantial portion of enzyme was inactive in vivo or that some activity was immediately lost upon cell lysis, despite our efforts to avoid the aeration or dilution of extracts. The same behavior was noted for the acnA mutant but not the acnB mutant, thus identifying AcnB as the partially inactive enzyme. Other workers have noted the same phenomenon (19).
FIG. 9.
Oxidants damage AcnB but not AcnA in vivo. (A) The aconitase activity of exponentially growing aerobic cultures was measured in scavenger-proficient or SOD− cells (solid bars). Aconitase activity was then reassayed after reactivation by iron and DTT (stippled bars). (B) Catalase-deficient mutants expressing AcnA (SMV10) or AcnB (SMV11) were concentrated anaerobically and exposed to 0 (open symbols) or 4 mM (filled symbols) H2O2. At indicated time points, aliquots were removed, H2O2 was scavenged with catalase, and cells were lysed for activity measurements. H2O2 measurements confirmed that H2O2 was not scavenged during the period of the cell exposure (data not shown). Circles, aconitase A; squares, aconitase B; triangles, fumarase.
To test whether oxidants could damage these enzymes in vivo, the aconitase activities were measured after mutant cells were stressed with superoxide or hydrogen peroxide. When SOD-deficient strains were cultured in air, about 85% of the wild-type activity was lost (Fig. 9A). The residual activity was mostly resistant to incubation with EDTA, indicating that it consisted primarily of AcnA. An acnA mutant, expressing only AcnB, lost most of its activity when SOD was removed by mutation. The activity could be restored to a level matching that of SOD-proficient cells when the extract was treated anaerobically with DTT and iron. Thus, AcnB is clearly sensitive to superoxide in vivo as well as in vitro.
In contrast, an acnB mutant retained about 22% of the wild-type activity, and this activity was not substantially diminished in a SOD-deficient background. AcnA is evidently resistant to superoxide in vivo. These patterns were also observed when catalase-deficient strains were exposed to hydrogen peroxide: AcnB, like FumB, was rapidly inactivated, but AcnA was resistant (Fig. 9B).
These data indicate that AcnB is the major aconitase in wild-type strains but is inactivated when iron levels fall or oxidants are present. AcnA is a minor aconitase that is distinguished by its resistance both to iron depletion and oxidation.
acnA mutations did not confer growth phenotypes.
We anticipated that the resistance of AcnA to oxidation might enable the TCA cycle to function even when oxidizing conditions inactivated most AcnB. To test this idea we monitored the growth of wild-type and acnA mutant strains in the presence of paraquat, a redox-cycling drug that generates superoxide and activates expression of acnA. The acnA mutants grew as well as their wild-type parent, in the absence or presence of paraquat, in LB, glucose-amino acids medium, and lactate medium (data not shown). Further, acnA sodA sodB mutants grew as rapidly as their sodA sodB counterparts in LB, in glucose-amino acids medium, and in the latter medium lacking the α-ketoglutarate family of amino acids (data not shown). In all of these media, acnA acnB null mutants grow poorly or not at all, due to the accumulation of intracellular citrate and/or their inability to generate α-ketoglutarate as a biosynthetic precursor. Thus, while SOD deficiency and paraquat treatment inactivated the majority of AcnB, enough residual activity remained that AcnA was not essential for function of the TCA cycle. This result would be expected if AcnB operates in unstressed cells far from its maximal turnover number (see Discussion).
AcnA moderates changes in citrate pools when AcnB is inactivated by superoxide.
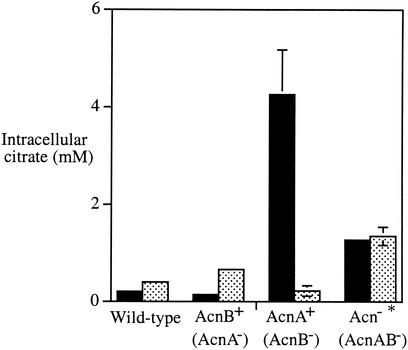
As an alternative approach to finding a phenotype for acnA, we monitored intracellular citrate levels to see whether oxidative damage to aconitase might cause a perceptible increase in the steady-state concentration of its substrate. Unstressed wild-type cells and acnA mutants contained low levels of citrate, whereas the concentration was much higher in acnB and acnA acnB mutants (Fig. 10). These data confirm that AcnB serves as the primary aconitase in vivo. Furthermore, substantial citrate was excreted into the medium by the AcnB-deficient strains (100 ± 17 μM [mean ± standard deviation]); a lesser amount was detected in the supernatants of wild-type and AcnA-deficient strains (20 ± 10 μM).
FIG. 10.
Intracellular accumulation of citrate. Intracellular citrate concentrations were determined in exponentially growing cells in the absence (solid bars) or presence (stippled bars) of 1 mM paraquat. Error bars represent the standard deviations of measurements in three independent cultures; where they are not visible, the deviation was <0.1 mM. The asterisk next to the acnA acnB strain indicates the recognition that this strain grows substantially more slowly than the others. The strains were W3110 (wild type) and its isogenic derivatives, JRG2789 (AcnB+), JRG3258 (AcnA+), and JRG3259 (Acn−).
When oxidative stress was imposed by paraquat, citrate levels rose moderately in the wild-type strain and more substantially in the acnA mutant (Fig. 10). Interestingly, they fell dramatically in the acnB mutant when paraquat was added, presumably because the SoxRS-mediated induction of AcnA compensated for the absence of AcnB. These results show that the oxidative inactivation of AcnB leads to an accumulation of intracellular citrate and indicate that this problem is ameliorated by the induction of AcnA.
DISCUSSION
Dehydratases that contain [4Fe-4S] clusters can be inactivated by any chemical oxidants that can penetrate into their active site and univalently oxidize the cluster. Previous work showed that aconitases of E. coli, yeast, and mammalian cells all lose activity in vitro and in vivo during oxidative stress (18, 20, 38), and in one study the steady-state aconitase activity of E. coli was used to infer the level of superoxide in that cell (19). However, more recently it was realized that E. coli actually contains two aconitases (24). In this work we have found that each aconitase exhibits behavior that is atypical of other members of the [4Fe-4S] dehydratase family.
Aconitase B evidently handles the majority of the substrate flux during routine growth. It is unusual in that it loses activity under nonoxidizing but iron-poor conditions, apparently because the catalytic iron dynamically equilibrates between the enzyme-bound and dissociated states. Aconitase A, like most dehydratases, is stable to iron depletion and, in purified form, is vulnerable to oxidative inactivation. However, inside the cell it is protected from small oxidants by an as-yet-unidentified factor.
Why do cells use unstable enzymes?
One adaptation of E. coli to superoxide stress is to replace labile dehydratases with stable isozymes. This strategy was first revealed in studies of fumarase C, a cluster-free dehydratase that is induced by SoxRS under conditions in which superoxide is abundant (35). The present results indicate that in vivo, aconitase A is another SoxRS-inducible, superoxide-resistant dehydratase. Interestingly, both aconitase A and fumarase C are also induced during entry into stationary phase as part of a general, preemptive antioxidant response that includes the induction of catalase (HPII), periplasmic SOD, exonuclease III, and other antioxidant enzymes (9).
Although the induction of resistant isozymes is a clever adaptation, it raises the basic question of why E. coli does not wholly dispense with the vulnerable enzymes and rely exclusively on their oxidant-resistant isozymes under all growth conditions. In fact, some organisms have already done this: mitochondria utilize a cluster-free fumarase analogous to fumarase C, and spinach employs a dihydroxyacid dehydratase with a stable [2Fe-2S] cluster rather than the vulnerable [4Fe-4S] type. Since E. coli already has stable alternatives to fumarase A and aconitase B in its genome but chooses to express them only during periods of oxidative stress, it seems that some benefit must follow from using the labile dehydratases as housekeeping enzymes.
The most obvious argument for retention of a labile enzyme is that it is kinetically superior to the alternatives. However, that appears not to be the case with either fumarase A or aconitase B, which have kcat/Km values similar to those of fumarase C or aconitase A, respectively (12, 28).
Furthermore, aconitase B has the additional irregularity of being substantially more abundant than is necessary to handle its substrate flux. Under the growth conditions examined here, the steady-state citrate concentration (0.2 mM) was far below the reported Km of AcnB (11 mM), so that AcnB functioned at only 2% of its maximal turnover. One consequence is that normal flux through the pathway should persist even when a substantial fraction of the enzyme is inactivated. This would explain our inability to detect any auxotrophy despite the imposition of superoxide stress. However, it reemphasizes the apparent wastefulness of this arrangement.
An explanation for this situation may be that the instability of the aconitase B cluster plays a useful purpose in iron metabolism. We have observed that aconitase B is demetallated when E. coli is starved for iron, and in a previous study Tang and Guest showed that demetallated AcnB binds its cognate mRNA (47). This behavior of AcnB resembles that of its cytosolic mammalian counterpart, which when demetallated binds hairpin structures (IREs) in the untranslated regions of transcripts that encode proteins involved in iron metabolism. In fact, demetallated AcnB binds to rabbit ferritin IRE in vitro. However, in contrast to that of AcnB, the aconitase activity of the metallated mammalian IRE-binding protein is not known to be physiologically significant. The aconitase of Bacillus subtilis is a closer functional homologue to AcnB, since it acts both as a catalytic aconitase and as an iron-dependent RNA-binding protein (1, 8). Its RNA-binding activity recognizes IRE-like elements in transcripts that encode cytochrome oxidase and iron-transport proteins.
It is commonly presumed that the demetallation of aconitases is precipitated by oxidation, but we observed that AcnB was inactivated upon iron depletion even under anaerobic conditions, when no oxidants were available to damage the cluster. This was true both of purified enzyme in vitro and of the enzyme in vivo. If apo-AcnB has evolved to regulate iron metabolism, nonoxidative demetallation would be a requirement, since much of the E. coli lifestyle occurs in the hypoxic intestine.
Interestingly, the loss of AcnB activity has the additional effect of causing the accumulation and excretion of citrate. Citrate is an excellent iron chelator, and E. coli is equipped with a transport system specific for iron-citrate chelates (7, 11). Therefore, the inactivation of AcnB upon iron starvation might trigger the release into the medium of a good iron siderophore.
Consistent with this idea, the synthesis of AcnA depends upon metallated Fur. This arrangement ensures that under conditions of iron limitation the accumulation of citrate is not relieved by the synthesis of AcnA. In contrast, the inactivation of AcnB by oxidants may be an unfortunate consequence of its cluster exposure and in itself serve no useful purpose. When AcnB inactivity is due to oxidative stress, AcnA induction relieves the metabolic block.
These schemes would rationalize the reliance of E. coli upon an unstable aconitase when a stable isozyme is available. However, while appealing, they have not yet been tested. Further, it is notable that E. coli has other oxidant-sensitive dehydratases whose retention thus far lacks a compelling explanation.
How does a factor protect AcnA without compromising its catalytic efficiency?
The active-site structure of aconitase A retains 17 of the 20 residues of aconitase B, indicating that it uses the same catalytic mechanism. Its unusual resistance to oxidants may be due to a tight association with the protective factor that has been detected in cell extracts. Efforts are under way to purify and characterize the factor.
Flint has pointed out that superoxide must be protonated in order to oxidize clusters (14). Thus, the absence of a proton-donating residue could, in principle, reduce the vulnerability of a cluster to this oxidant. However, AcnA is also resistant to H2O2 and ferricyanide, which do not require protonation. These are also strong oxidants that are unlikely to be deterred by a moderate shift in cluster potential. Therefore, the simplest model for the resistance of factor-bound AcnA is that a bound factor blocks the penetration of small oxidants into the active site. At the same time, however, substrate continues to gain access: we observed no decrease in the turnover of the purified enzyme when the protective factor was added. Detailed kinetic analysis awaits the purification of the factor. It remains to be determined, of course, whether the factor that protected AcnA in vitro is also that which protects it in vivo.
Recently, Gralnick and Downs suggested that Salmonella enterica serovar Typhimurium may contain a protein that protects labile iron-sulfur clusters (22). They observed that mutations that elevate the expression of yggX have the effect of improving the oxidant resistance of several iron-sulfur-cluster enzymes in vivo, including aconitase, and also reduce the rate of spontaneous mutation. The latter effect could plausibly result from a reduced rate of iron leakage from damaged enzymes. The mechanism of yggX action has not been established. We find that the factor which protects AcnA in E. coli seems not to stabilize any of the other enzymes we have tested, including AcnB, fumarase A, and 6-phosphogluconate dehydratase. E. coli has a yggX homologue, however, and the possibility that it encodes the AcnA protective factor will have to be tested genetically.
Interestingly, two other oxidatively unstable enzymes from other bacteria have been shown to be protected by physical exclusion of oxidants. The iron-sulfur clusters of Azotobacter vinelandii nitrogenase are thought to be shielded during periods of oxygen exposure by Shethna protein (39). In this situation, however, the protected nitrogenase is catalytically inactive, regaining function only when low oxygen levels are restored and the Shethna protein is deactivated. The pyruvate:ferredoxin oxidoreductase (PFOR) of Desulfovibrio africaans is more oxygen stable than most PFOR enzymes, apparently due to the presence of an extra domain that is situated to occlude the active-site [4Fe-4S] cluster (44). Mutants that lack the domain exhibit the hypersensitivity to oxygen that is typical of PFOR enzymes. This domain, like the protective factor of AcnA, does not block enzyme activity.
The instability of aconitase B complicates biochemical assays.
Two features of AcnB—its dependence upon dissolved iron and its high Km for citrate—make it a tricky enzyme to work with in vitro. Conventional assays with citrate as a substrate typically yield nonlinear reaction rates, because even 30 mM citrate cannot fully saturate and protect its active site against oxidation and/or demetallation. Its high Km also solves the puzzle (19) of why oxidants can damage AcnB in vivo, despite the presence of substrate.
Demetallation in dilute extracts likely has compromised some reports of aconitase activity. In our hands, AcnB could be reliably assayed only in concentrated (or iron-supplemented) anaerobic extracts with isocitrate as a substrate. In fact, the initial purification of aconitase activity from E. coli resulted in the recovery of the minor enzyme, AcnA, rather than AcnB, probably because the latter was rapidly inactivated during the attempt (46). Similarly, the aconitase isolated by Hausladen and Fridovich for measurements of superoxide sensitivity was presumably AcnA, as indicated by its resistance to EDTA (26). The distinction between AcnA and AcnB may also be at the root of discrepancies in the literature (16) regarding the sensitivity of E. coli aconitase to nitric oxide.
Acknowledgments
We are grateful to John Guest, University of Sheffield, for providing strains and counsel.
This work was supported by grant GM49060 from the National Institutes of Health and grant 060940 from the Wellcome Trust.
REFERENCES
- 1.Alen, C., and A. L. Sonenshein. 1999. Bacillus subtilis aconitase is an RNA-binding protein. Proc. Natl. Acad. Sci. USA 96:10412-10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauchamp, C., and I. Fridovich. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44:276-287. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, B., M. J. Gruer, J. R. Guest, and A. J. Thomson. 1995. Spectroscopic characterisation of an aconitase (AcnA) of Escherichia coli. Eur. J. Biochem. 233:317-326. [DOI] [PubMed] [Google Scholar]
- 4.Blank, L., J. Green, and J. R. Guest. 2002. AcnC of Escherichia coli is a 2-methylcitrate dehydratase (PrpD) that can use citrate and isocitrate as substrates. Microbiology 148:133-146. [DOI] [PubMed] [Google Scholar]
- 5.Carlioz, A., and D. Touati. 1986. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 5:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, E., and B. Weiss. 1987. Endonuclease IV of Escherichia coli is induced by paraquat. Proc. Natl. Acad. Sci. USA 84:3189-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke, T. E., L. W. Tari, and H. J. Vogel. 2001. Structural biology of bacterial iron uptake systems. Curr. Top. Med. Chem. 1:7-30. [DOI] [PubMed] [Google Scholar]
- 8.Craig, J. E., M. J. Ford, D. C. Blaydon, and A. L. Sonenshein. 1997. A null mutation in the Bacillus subtilis aconitase gene causes a block in SpoOA-phosphate-dependent gene expression. J. Bacteriol. 179:7351-7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenstark, A., M. J. Calcutt, M. Becker-Hapak, and A. Ivanova. 1996. Role of Escherichia coli rpoS and associated genes in defense against oxidative damage. Free Radic. Biol. Med. 21:975-993. [DOI] [PubMed] [Google Scholar]
- 10.Farr, S. B., R. D'Ari, and D. Touati. 1986. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc. Natl. Acad. Sci. USA 83:8268-8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 12.Flint, D. H., and R. M. Allen. 1996. Iron-sulfur proteins with nonredox functions. Chem. Rev. 96:2315-2334. [DOI] [PubMed] [Google Scholar]
- 13.Flint, D. H., M. H. Emptage, and J. R. Guest. 1992. Fumarase A from Escherichia coli: purification and characterization as an iron-sulfur cluster containing enzyme. Biochemistry 31:10331-10337. [DOI] [PubMed] [Google Scholar]
- 14.Flint, D. H., J. F. Tuminello, and M. H. Emptage. 1993. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J. Biol. Chem. 268:22369-22376. [PubMed] [Google Scholar]
- 15.Fraenkel, D. G., and B. L. Horecker. 1964. Pathways of d-glucose metabolism in Salmonella typhimurium: a study of a mutant lacking phophoglucose isomerase. J. Biol. Chem. 239:2765-2771. [PubMed] [Google Scholar]
- 16.Gardner, P. R., G. Constantino, C. Szabo, and A. L. Salzman. 1997. Nitric oxide sensitivity of aconitases. J. Biol. Chem. 272:25071-25076. [DOI] [PubMed] [Google Scholar]
- 17.Gardner, P. R., and I. Fridovich. 1991. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J. Biol. Chem. 266:1478-1483. [PubMed] [Google Scholar]
- 18.Gardner, P. R., and I. Fridovich. 1991. Superoxide sensitivity of the Escherichia coli aconitase. J. Biol. Chem. 266:19328-19333. [PubMed] [Google Scholar]
- 19.Gardner, P. R., and I. Fridovich. 1992. Inactivation-reactivation of aconitase in Escherichia coli: a sensitive measure of superoxide radical. J. Biol. Chem. 267:8757-8763. [PubMed] [Google Scholar]
- 20.Gardner, P. R., I. Raineri, L. B. Epstein, and C. W. White. 1995. Superoxide radical and iron modulate aconitase activity in mammalian cells. J. Biol. Chem. 270:13399-13405. [DOI] [PubMed] [Google Scholar]
- 21.Gort, A. S., and J. A. Imlay. 1998. Balance between endogenous superoxide stress and antioxidant defenses. J. Bacteriol. 180:1402-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gralnick, J., and D. Downs. 2001. Protection from superoxide damage associated with an increased level of the YggX protein in Salmonella enterica. Proc. Natl. Acad. Sci. USA 98:8030-8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg, J. T., P. Monach, J. H. Chou, P. D. Josephy, and B. Demple. 1990. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in E.coli. Proc. Natl. Acad. Sci. USA 87:6181-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruer, M. J., and J. R. Guest. 1994. Two genetically-distinct and differentially-regulated aconitases (AcnA and AcnB) in Escherichia coli. Microbiology 140:2531-2541. [DOI] [PubMed] [Google Scholar]
- 25.Hassan, H. M., and I. Fridovich. 1977. Regulation of the synthesis of superoxide dismutase in Escherichia coli: induction by methyl viologen. J. Biol. Chem. 252:7667-7672. [PubMed] [Google Scholar]
- 26.Hausladen, A., and I. Fridovich. 1994. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J. Biol. Chem. 269:29405-29408. [PubMed] [Google Scholar]
- 27.Imlay, J. A., and I. Fridovich. 1991. Assay of metabolic superoxide production in Escherichia coli. J. Biol. Chem. 266:6957-6965. [PubMed] [Google Scholar]
- 28.Jordan, P. A., Y. Tang, A. J. Bradbury, A. J. Thomson, and J. R. Guest. 1999. Biochemical and spectroscopic characterization of Escherichia coli aconitases (AcnA and AcnB). Biochem. J. 344:739-746. [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy, M. C., C. D. Stout, and H. Beinert. 1996. Aconitase as Fe-S protein, enzyme, and iron regulatory protein. Chem. Rev. 96:2335-2374. [DOI] [PubMed] [Google Scholar]
- 30.Keyer, K., A. S. Gort, and J. A. Imlay. 1995. Superoxide and the production of oxidative DNA damage. J. Bacteriol. 177:6782-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keyer, K., and J. A. Imlay. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA 93:13635-13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keyer, K., and J. A. Imlay. 1997. Inactivation of dehydratase [4Fe-4S] clusters and disruption of iron homeostasis upon cell exposure to peroxynitrite. J. Biol. Chem. 272:27652-27659. [DOI] [PubMed] [Google Scholar]
- 33.Klausner, R. D., and T. A. Rouault. 1993. A double life: cytosolic aconitase as a regulatory RNA binding protein. Mol. Cell. Biol. 4:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo, C. F., T. Mashino, and I. Fridovich. 1987. α,β-dihydroxyisovalerate dehydratase: a superoxide-sensitive enzyme. J. Biol. Chem. 262:4724-4727. [PubMed] [Google Scholar]
- 35.Liochev, S. I., and I. Fridovich. 1992. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc. Natl. Acad. Sci. USA 89:5892-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liochev, S. I., and I. Fridovich. 1994. The role of superoxide in the production of hydroxyl radical: in vitro and in vivo. Free Radic. Biol. Med. 16:29-33. [DOI] [PubMed] [Google Scholar]
- 37.Loewen, P. C. 1984. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J. Bacteriol. 157:622-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longo, V. D., L.-L. Liou, J. S. Valentine, and E. B. Gralla. 1999. Mitochondrial superoxide decreases yeast survival in stationary phase. Arch. Biochem. Biophys. 365:131-142. [DOI] [PubMed] [Google Scholar]
- 39.Maier, R. J., and F. Moshiri. 2000. Role of the Azotobacteri vinelandii nitrogenase-protective Shethna protein in preventing oxygen-mediated cell death. J. Bacteriol. 182:3854-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maloy, S. R., and W. D. Nunn. 1981. Selection for loss of tetracycline resistance by Escherichia coli. J. Bacteriol. 145:1110-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Messner, K. R., and J. A. Imlay. 1999. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 274:10119-10128. [DOI] [PubMed] [Google Scholar]
- 42.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Misra, H. P., and I. Fridovich. 1977. Superoxide dismutase: “positive” spectrophotometric assays. Anal. Biochem. 79:553-560. [DOI] [PubMed] [Google Scholar]
- 44.Pieulle, L., V. Magro, and E. C. Hatchikian. 1997. Isolation and analysis of the gene encoding the pyruvate-ferredoxin oxidoreductase of Desulfovibrio africanus, production of the recombinant enzyme in Escherichia coli, and effect of carboxy-terminal deletions on its stability. J. Bacteriol. 179:5684-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pomposiello, P. J., M. H. Bennik, and B. Demple. 2001. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J. Bacteriol. 183:3890-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prodromou, C., M. J. Haynes, and J. R. Guest. 1991. The aconitase of Escherichia coli: purification of the enzyme and molecular cloning and map location of the gene (acn). J. Gen. Microbiol. 137:2505-2515. [DOI] [PubMed] [Google Scholar]
- 47.Tang, Y., and J. R. Guest. 1999. Direct evidence for mRNA binding and post-transcriptional regulation by Escherichi coli aconitases. Microbiology 145:3069-3079. [DOI] [PubMed] [Google Scholar]
- 48.Tsaneva, I. R., and B. Weiss. 1990. soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. J. Bacteriol. 172:4197-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]