Abstract
Escherichia coli is a major cause of enteric/diarrheal diseases, urinary tract infections, and sepsis. E. coli K1 is the leading gram-negative organism causing neonatal meningitis, but the microbial basis of E. coli K1 meningitis is incompletely understood. Here we employed comparative genomic hybridization to investigate 11 strains of E. coli K1 isolated from the cerebrospinal fluid (CSF) of patients with meningitis. These 11 strains cover the majority of common O serotypes in E. coli K1 isolates from CSF. Our data demonstrated that these 11 strains of E. coli K1 can be categorized into two groups based on their profile for putative virulence factors, lipoproteins, proteases, and outer membrane proteins. Of interest, we showed that some open reading frames (ORFs) encoding the type III secretion system apparatus were found in group 2 strains but not in group 1 strains, while ORFs encoding the general secretory pathway are predominant in group 1 strains. These findings suggest that E. coli K1 strains isolated from CSF can be divided into two groups and these two groups of E. coli K1 may utilize different mechanisms to induce meningitis.
The mortality and morbidity associated with neonatal gram-negative bacillary meningitis remain significant despite advances in antimicrobial chemotherapy and supportive care. The mortality rates have ranged between 5 and 50%, with the majority (30 to 50%) of surviving infants manifesting neurological sequelae (15, 25). A major contributing factor is the incomplete understanding of the pathogenesis of this disease.
Escherichia coli is the most common gram-negative organism that causes meningitis during the neonatal period, and E. coli strains possessing the K1 capsular polysaccharide are predominant (∼80%) among isolates from neonatal E. coli meningitis (26, 44, 58). At present, a few E. coli K1 strains isolated from cerebrospinal fluid (CSF) have been used to study the microbial basis of meningitis, but it is unclear whether the information derived from these E. coli K1 strains is relevant to other E. coli K1 strains isolated from CSF. For example, CNF1 (cytotoxic necrotizing factor 1) and IbeA have been shown to contribute to the pathogenesis of meningitis in strain RS218, i.e., invasion of human brain microvascular endothelial cells (HBMEC) in vitro and traversal of the blood-brain barrier (BBB) in vivo (35, 40), but it is unknown whether CNF1 and IbeA exist and play the same roles in other E. coli K1 strains isolated from CSF. Therefore, a better knowledge of the distribution pattern of known and putative virulence factors will improve our understanding of meningitis caused by E. coli K1.
The distribution of some known E. coli virulence factors has been reported in avian-pathogenic E. coli (APEC) (37), uropathogenic E. coli (21), enteropathogenic E. coli (EPEC), and enterohemorrhagic E. coli (EHEC) (50). For example, the distribution of these known and putative virulence factors has been used to determine phylogenetic relationships among meningitis-causing E. coli isolates (7, 39, 43). However, these results were based on analysis of relatively small numbers of virulence factors. To study the microbial basis of meningitis-causing E. coli K1 on the genome level, we constructed a comprehensive E. coli microarray which covers most of the known E. coli genes to examine the distribution of putative virulence factors and compare the genome contents of 11 representative strains of CSF-derived E. coli K1 belonging to serotypes common in meningitis (i.e., O18, O7, O1, O16, O12, and O45).
Comparative genomic hybridization (CGH) developed based on microarrays has shown differences in the contents of genes and genomic islands in closely related strains or species with different host ranges and virulence characteristics (18, 31, 59). CGH has also been used to study the distribution of specific genes among bacterial strains without the requirement of genome sequences (68). In this study, we employed CGH to investigate 11 representative strains of E. coli K1 isolated from the CSF of patients with meningitis. Based on genome profiles, our results revealed that the 11 strains can be divided into two groups and the two groups may utilize different mechanisms to cause meningitis.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study are listed in Table 1. Strains S88 and S95 were obtained from E. Bingen (8), and IHE3034 was obtained from T. Korhonen (49). The remaining E. coli strains were described previously (14, 42). Bacteria were grown overnight at 37°C in Luria broth.
TABLE 1.
E. coli K1 strains included in this studya
| Strain | Serotype | Origin | Reference(s) |
|---|---|---|---|
| RS218 | O18:K1:H7 | North America | 32, 40, 64 |
| C5 | O18:K1:H7 | North America | 41 |
| IHE3034 | O18:K1:H7 | Finland | 49 |
| EC10 | O7:K1 | North America | 42 |
| A90 | O1:K1:H7 | Finland | 42 |
| RS167 | O16:K1:H6 | North America | 42 |
| RS168 | O1:K1:H− | North America | 42 |
| S88 | O45:K1 | France | 8 |
| S95 | O45:K1 | France | 8 |
| E253 | O12:K1 | North America | 14 |
| E334 | O12:K1 | North America | 14 |
All strains were judged virulent based on their ability to cause bacteremia and meningitis in 5-day-old rats.
Microarray descriptions.
A total of 8,144 50-mer oligonucleotides from E. coli and 95 negative-control oligonucleotides from human and Arabidopsis thaliana were spotted in replicate onto aminosilane slides. The oligonucleotides that are targeting backbone genes in E. coli genomes were derived from a commercially designed oligonucleotide set, which covers every open reading frame (ORF) present in E. coli strain MG1655, and EHEC O157:H7 strains EDL933 and Sakai (all together 6,268 50-mers). The extraintestinal pathogenic E. coli-specific (ExPEC) oligonucleotide probes were derived from uropathogenic E. coli strain CFT073 and meningitis-causing E. coli strain (RS218 and C5) sequences that were different from and/or absent in E. coli backbone (1,876 50-mers). In principle, oligonucleotides from the commercial source were constructed in accumulative fashion, i.e., oligonucleotides targeting every ORF present in MG1655 were constructed and O157:H7-specific probes were added subsequently. Similarly, ExPEC-specific 50-mer oligonucleotide probes were designed and constructed to adapt the existing oligonucleotide set to use in meningitis-causing or uropathogenic E. coli. The majority of conserved backbone ORFs or E. coli strain-specific ORFs were targeted with single probes. ORFs that were shared among different E. coli strains but appeared to be hypervariable were usually targeted with two or more oligonucleotide probes.
DNA isolation, labeling, and hybridization.
E. coli strains were grown overnight in brain heart infusion broth (64) at 37°C with shaking. A 1.5-ml amount of overnight culture was centrifuged for 5 min at 4°C at high speed in a tabletop centrifuge (Eppendorf, Westbury, NY). Supernatants were discarded, and cell pellets were resuspended in an equal volume of TE buffer (10 mM Tris, 1 mM EDTA; pH 8.0). Briefly, genomic DNA was isolated and labeled as described previously with a modification (2, 68). Genomic DNA was digested completely with EcoRV (New England Biolabs, Beverly, MA) and purified by using the QIAquick PCR purification kit (QIAGEN, Valencia, CA). Digested DNA (2 μg) was mixed with 4 μg random hexamer (Invitrogen, Carlsbad, CA) and 5 μl NEB buffer 2 (New England Biolabs, Beverly, MA) in a total volume of 44 μl and then denatured at 95°C for 10 min, followed by snap cooling on ice. Five microliters of deoxynucleoside triphosphate mixture (5 mM dATP, 5 mM dCTP, 5 mM dGTP, 1 mM dTTP, 4 mM aminoallyl-dUTP) and 1 μl Klenow enzyme (New England Biolabs) were added, and the labeling was carried out for 12 h at 37°C. The products were purified by using the QIAquick PCR purification kit (QIAGEN), eluted in 10 μl water, and dissolved with either Cy3 or Cy5 monofunctional N-hydroxysuccinimide ester (Amersham Biosciences, Piscataway, NJ) in 2 μl dimethyl sulfoxide. Two microliters of dye solution and 1 μl of labeling buffer (1 M sodium bicarbonate, pH 9.3) were added to the purified amine-modified DNA. The mixture was incubated in the dark at room temperature for 1 h. The labels were purified by using the QIAquick PCR purification kit (QIAGEN). The purified Cy3- and Cy5-coupled labels were combined and concentrated by centrifugation in a spin filter (Nanosep; molecular weight cutoff, 30,000; Pall, Ann Arbor, MI). The concentrated DNA was resuspended in 50 μl hybridization buffer (Pronto! universal hybridization kit; Corning, Corning, NY). The hybridization mixture was then denatured at 95°C for 2 min. The mixture was applied onto the array under a LifterSlip coverslip (Erie Scientific, Portsmouth, NH). The assembled slide was placed in a hybridization chamber (Corning, Park Acton, MA) and incubated at 42°C for 16 to 18 h. Following hybridization, slides were extensively washed for 1 min in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate at 42°C, 5 min in 2× SSC-0.1% sodium dodecyl sulfate at 42°C, 10 min in 1× SSC at room temperature, and 2 min in 0.1× SSC at room temperature twice. Each experiment was run as a competitive hybridization by using Cy3-labeled DNA from one of the 10 strains and Cy5-labeled DNA from strain RS218. Then, experiments were repeated by reversing dyes.
Array scanning and analysis.
Arrays were scanned by using a GenePix 4000B microarray scanner (Axon Instruments, Fremont, CA) with 100% scan power and photomultiplier tube voltage set for each array to minimize saturated pixels and approximately equalize signal intensities in the two channels. Image processing and data extraction were performed by using GenePix Pro 6.0 (Axon Instruments). Spots with high background fluorescence and slide abnormalities were excluded from analysis. Firstly, the medians of spot intensities (MIs) of all slides were calculated. Secondly, the medians of spot intensities of two channels on each slide were normalized to the average of MIs of all slides. ORFs with intensity less than the negative-control value (the median intensities of 95 negative-control oligonucleotides) are considered “absent,” and ORFs with intensities greater than the average of MIs (validated by PCR and in silico analysis) are considered “present.” If both channels could not be determined by the above criteria, these ORFs were considered “divergent.” For remaining ORFs, if channel A showed “absent” and the ratio of intensities (B/A) was less than 3.0, these ORFs in channel B were considered “absent,” and if the ratio was greater than 3.0, then these ORFs were considered “divergent.” If channel A showed “present” and the ratio of intensities (B/A) was less than 0.33, these ORFs in channel B were considered “absent,” and if the ratio was greater than 0.33, then these ORFs were considered “present.”
Hierarchical clustering.
Data imported from GenePix were manipulated and clustered, using established algorithms implemented in the software program Cluster (19). Average linkage clustering with centered correlation was used. TreeView software generated visual representations of clusters (54).
PCR confirmation of microarray data.
Several selected genes were examined by PCR to confirm our microarray data. Primers were designed by Primer 3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). PCR primers are listed in Table 2.
TABLE 2.
Sequences of PCR primers used in this study
| Gene | Sequence
|
|
|---|---|---|
| Forward | Reverse | |
| cnf1 | GGACTCGAGGTGGTGGTCTA | CGTTGATGGCTCAGGAAAAT |
| aslA | GCGTGATGTTCATGTCAACC | ATCCGCCAGATCTACAATGC |
| fyuA | GGCTATACCACCGCTGAAAC | ACCCGGTTACCGTGATACAA |
| hlyA | CTTAGGAAAGGCAGGCAGTG | ACTTATCGGCAATGGACAGG |
| hlyE | CCCGCAGCAATAGAATAGGA | CCGCAGATGGAGCATTAGAT |
| ibeA | AGAAACGGCAAAATCAATGG | ACTCGGTGACCGTACTCCAG |
| iha | TGCAGGCTGACAGAATCATC | CCGGCTATGAGAAAAAGCTG |
| iucA | ACCACTCTCTCCCGGCTTAT | GTAGAGGGAACGGGAAGAGG |
Triplex PCR for phylogenetic grouping.
The above 11 representative strains of E. coli K1 were also examined for E. coli phylogenetic group by using a combination of two genes (chuA and yjaA) and an anonymous DNA fragment (TspE4C2). PCR was carried out as described previously (11).
RESULTS
Overview of 11 strains.
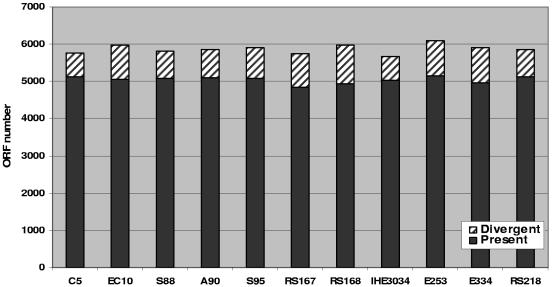
We selected 11 strains of E. coli K1 which were isolated from the CSF of patients with meningitis. These strains included serotypes that are shown to be common in E. coli meningitis, i.e., O1, O7, O12, O16, and O18. In addition, E. coli K1 isolates belonging to a new O45:K1 group have been included, which have been shown to be predominant in neonates with E. coli meningitis from France (8). After data analysis, the ORFs of these 11 representative strains have been estimated. The total ORF numbers in the genomes of the 11 strains are close to each other, from 4,852 (strain RS167) to 5,147 (strain E253) ORFs (Fig. 1). Some strains may harbor specific ORFs which may not be detectable by our microarray, and thus, the actual ORF numbers may be higher than those shown in Fig. 1. All the microarray data are provided in Table S1 in the supplemental material. To validate our microarray data, 32 ORFs which have been sequenced in either strain RS218 or strain C5 were chosen for in silico analysis (see Table S2 in the supplemental material). The results showed consistency between microarray and in silico analysis, indicating that our microarray data are reliable.
FIG. 1.
Genome of 11 representative strains of E. coli K1. The total ORFs were calculated based on microarray hybridization. Spots were excluded from analysis because of high local background fluorescence and slide abnormalities. The determination of cutoff values has been described in Materials and Methods.
Genetic relationship between strains.
To evaluate the relationship among these 11 representative strains, we performed hierarchical clustering, using the data from comparative genomic hybridization (Fig. 2). Clustering revealed that some E. coli K1 strains with the same O serotypes are closely related to each other. For example, O18:K1:H7 strains RS218, IHE3034, and C5 are closely related to each other and the two O45 strains, S88 and S95, are most closely related to each other. Clustering analysis also linked E. coli strains with different O serotypes. For example, strains EC10 (O7), RS168 (O1), and E253 (O12) were clustered together. Strain RS168, which belongs to O1, was found to be close to strain EC10 (O7), not to strain A90 (O1). Similarly, strains E252 and E334, belonging to O12, were clustered into different groups. Overall, strains EC10, RS168, and E253 represent a distinct cluster and appear to be most distantly related to the RS218 group. Here, based on genome similarity, we named the cluster which contains strains RS218, IHE3034, C5, RS167, A90, S88, S95, and E334 as group 1 and the other strains as group 2.
FIG. 2.
Clustering of 11 representative meningitis-causing E. coli K1 strains based on microarray data. Data imported from GenePix were manipulated and clustered, using established algorithms implemented in the software program Cluster (19). Average linkage clustering with centered correlation was used. TreeView software generated visual representations of clusters (54).
Phylogenetic grouping.
All 11 representative strains of E. coli K1 were classified as to E. coli phylogenetic group by using a combination of two genes (chuA and yjaA) and an anonymous DNA fragment (TspE4C2) (11). The triplex PCR results showed that strains RS218, IHE3034, C5, A90, RS167, E334, S88, and S95 belong to group B2 while strains EC10 and RS168 are in group D and strain E253 is in group A.
Specific virulence factors.
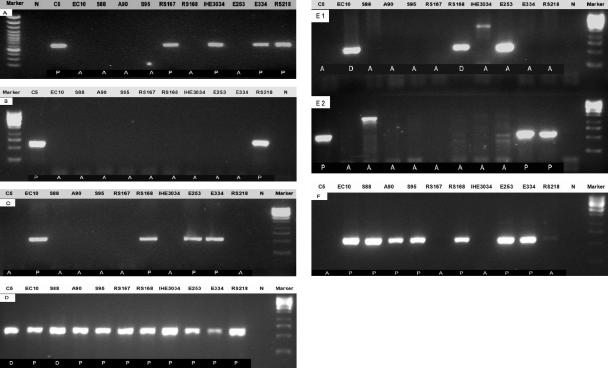
We next examined the distribution of selected E. coli virulence factors printed on a microarray among these 11 representative E. coli K1 strains based on the available information that may be relevant to meningitis (Table 3). The gene ibeA, previously identified from RS218 to contribute to the invasion of HBMEC in vitro and traversal of the blood-brain barrier in vivo (35), was also found to be present in strains C5, RS167, IHE3034, and E334 by microarray analysis. This was confirmed by PCR analysis (Fig. 3A). CNF1 is a 115-kDa protein toxin produced by extraintestinal E. coli strains which include uropathogenic and meningitis-causing E. coli (9). A cnf1 mutant had decreased virulence in a mouse model of ascending urinary tract infection compared to the isogenic cnf1+ strain (57). We have previously shown that CNF1 is a virulence factor contributing to E. coli K1 invasion of HBMEC in vitro and traversal of the BBB in vivo (40). cnf1 was found to be present in only 2 of the 11 strains (C5 and RS218) by both microarray and PCR analysis (Fig. 3B). The iron-regulated gene homologue adhesin (iha), an E. coli O157:H7 outer membrane protein, was shown to confer the adherence phenotype upon nonadherent laboratory E. coli and shown to be a virulence factor in uropathogenic E. coli (38). iha encodes a 67-kDa protein in E. coli O157:H7 similar to iron-regulated gene A (IrgA) of Vibrio cholerae (22). Microarray and PCR analysis showed that iha is present in all group 2 strains (EC10, RS168, and E253) and in only one group 1 strain, E334 (Fig. 3C). The yersiniabactin receptor (fyuA) gene was the most highly prevalent iron utilization system in enteroaggregative E. coli and APEC (20, 53). fyuA was found to be uniformly present in all 11 representative strains by microarray analysis, which was confirmed by PCR (Fig. 3D). The iuc (iron uptake chelate) gene locus encodes enzymes necessary for synthesis of the siderophore aerobactin, whereas the iutA (iron uptake transport) gene product represents the TonB-dependent outer membrane receptor for ferric aerobactin. The first step in aerobactin synthesis is hydroxylation of l-lysine by IucD, whereas IucB is responsible for acetylation of N-hydroxylysine. Two N-acetyl-N-hydroxylysine molecules are then attached to the carboxylic groups of citric acid by IucC and probably IucA, resulting in aerobactin (16, 17). iuc was shown to be present in all strains, except for strains C5, RS167, IHE3034, and RS218, and PCR verified the microarray data (Fig. 3F). However, iutA is present in all group 2 strains (EC10, RS168, and E253) and one group 1 strain, E334. Hemolysin production was described in approximately 23% of the E. coli strains associated with neonatal meningitis (43), and our microarray showed the presence of hlyABCD in strains C5, E334, and RS218 but not in others (Table 3). PCR analysis of hlyA also confirmed the microarray result but revealed a different amplification product (about 1 kb compared with the expected 550-bp product) in strain S88 (Fig. 3E2). This 1-kb band from strain S88 was sequenced, and BLAST showed that it is similar to ORF Z1789 (O157 EDL933), which encodes a putative AraC-type regulatory protein. The distribution of hlyE is completely different from that of hlyABCD. Only group 2 strains EC10 and RS168 showed the potential presence of hlyE by microarray analysis (Table 3). However, PCR and sequencing analysis showed that all group 2 strains (EC10, RS168, and E253) harbor hlyE (Fig. 3E1). These differences might be related to a design problem with an oligonucleotide from MWG (High Point, NC).
TABLE 3.
Distribution of selected E. coli virulence factors among 11 E. coli K1 strainsa
| ORF | Function | Presence of virulence factor in strainb:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RS218 | C5 | IHE3034 | S88 | S95 | A90 | RS167 | E334 | EC10 | RS168 | E253 | ||
| cnf1 | Cytotoxic necrotizing factor 1 | + | + | − | − | − | − | − | − | − | − | − |
| aslA | Arylsulfatase-like gene | + | +/− | +/− | +/− | +/− | +/− | + | + | +/− | +/− | + |
| fhuA | Ferrichrome-iron receptor precursor; fhuA | + | + | + | + | + | + | + | + | +/− | + | + |
| fyuA | Pesticin/yersiniabactin receptor protein | + | +/− | + | +/− | + | + | + | + | + | + | + |
| hlyABCD | Hemolysin | + | + | − | − | − | − | − | + | − | − | − |
| hlyE | Hemolysin E | − | − | − | − | − | − | − | − | +/− | +/− | − |
| ibeA | Invasin IbeA | + | + | + | − | − | − | + | + | − | − | − |
| ibeB | Invasin IbeB | + | + | + | + | + | + | + | + | + | + | + |
| iha | Adhesin | − | − | − | − | − | − | − | + | + | + | + |
| iroN | Ferric uptake protein | + | + | + | + | + | + | + | + | − | − | − |
| iucABCD | IucABCD protein | − | − | − | + | + | + | − | + | + | + | + |
| iutA | IutA protein | − | − | − | − | − | − | − | + | + | + | + |
| malX | PTSc family enzyme IIC | + | + | + | + | + | + | + | + | + | + | + |
| ompA | Outer membrane protein | + | + | + | + | + | + | + | + | + | + | + |
| sfa/foc | S fimbria | + | + | + | − | − | − | − | − | − | − | − |
| sfaG | F1C minor fimbria | + | + | + | +/− | +/− | +/− | +/− | +/− | + | + | + |
| papA | P fimbria | + | − | − | + | + | + | +/− | + | + | + | + |
| hecA | Hemolysin/hemagglutinin-like protein | + | + | − | − | − | − | − | − | − | − | − |
| flu | Antigen 43 | + | + | + | + | + | + | +/− | + | − | + | + |
| hek | Adhesin/virulence factor | + | + | − | +/− | +/− | +/− | − | +/− | +/− | +/− | +/− |
| traJ | TraJ protein | + | + | − | − | − | − | + | + | − | − | + |
| neu | K1 capsule | + | + | + | + | + | + | + | + | + | + | + |
| sitABCD | Sit proteins | + | + | + | + | + | + | + | + | + | + | + |
Only microarray data are given, not PCR confirmation results.
+, present; −, absent; +/−, divergent.
PTS, phosphotransferase system.
FIG. 3.
PCR verification. Selected genes were examined by PCR to confirm microarray data. Primers listed in Table 2 were designed by Primer 3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). PCR programs for each selected gene were optimized by using the same amount of genomic DNA as template. Microarray data for selected genes (P, present; A, absent; D, divergent) are shown at the bottom of each panel. (A) ibeA; (B) cnf1; (C) iha; (D) fyuA; (E1) hlyE; (E2) hlyA; (F) iucA.
Lipoprotein.
Lipoprotein is a major component of the outer membrane of members of the family Enterobacteriaceae. Lipoprotein induces proinflammatory cytokine production in macrophages and lethal shock in lipopolysaccharide-responsive and nonresponsive mice (67). Bacterial lipoproteins comprise a unique set of proteins modified at their amino-terminal cysteines by the addition of N-acyl and S-diacyl glyceryl groups (67). In E. coli, this lipid serves to anchor these proteins to the inner or outer membrane so that they can function at the lipid-aqueous interface. These proteins can be identified by the presence of a leader with a common consensus sequence (10). The leader is typically between 15 and 40 amino acid residues in length and has at least one arginine or lysine in the first seven residues. The leader is cleaved by signal peptidase II on the amino-terminal side of the cysteine residue, which is then enzymatically modified (67). Our data revealed that about 100 lipoproteins are present in E. coli K-12 (46). Fifty-four ORFs which encode lipoproteins or putative lipoproteins were printed on our microarray, and about half of them (20 of 54) are shown to be present in all 11 representative strains of E. coli K1 (Table 4). For example, cutF (nlpE), encoding an outer membrane lipoprotein, is involved in copper transport in E. coli, and a mutation of cutF results in an increased copper sensitivity in E. coli (28). cutF is shown to be present in all 11 E. coli K1 strains. rlpA and rlpB, encoding two lipoproteins, are located in the leuS-dacA region (15 min) on the E. coli chromosome (63). A truncated rlpA gene in E. coli was able to rescue a conditionally lethal mutation in the prc gene (involved in C-terminal processing of penicillin-binding protein 3) (6). The prc mutants are sensitive to heat and osmotic stress (29). rlpA is shown to be present in all 11 E. coli K1 strains (Table 4). LolCDE, an ATP-binding cassette transporter, releases outer membrane-specific lipoproteins from the inner membrane to form a complex between the released lipoproteins and the periplasmic molecular chaperone LolA (51). LolA was shown to release other outer membrane lipoproteins such as Pal, NlpB, Slp, and RlpA, whereas inner membrane lipoproteins AcrA and NlpA were not released even in the presence of LolA (69), indicating that LolA plays a critical role in the sorting of lipoproteins. lolA is shown to be present in all 11 E. coli K1 strains (Table 4). An outer membrane lipoprotein encoded by nlpI may be important for the process of cell division in E. coli K-12 and has been shown to be involved in adherence to and invasion of intestinal epithelial cells by E. coli strain LF82 (5, 52). This nlpI gene is also found to be present in all 11 E. coli K1 strains (Table 4). Of interest, aec24, encoding a hypothetical lipoprotein, is shown to be present in group 1 strains but absent in group 2 strains.
TABLE 4.
Distribution of lipoproteins among meningitis-causing E. coli K1 strainsa
| ORF | Function | Presence of ORF in strainb:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RS218 | C5 | IHE3034 | S88 | S95 | A90 | RS167 | E334 | EC10 | RS168 | E253 | ||
| aec24 | Hypothetical lipoprotein | + | + | + | + | + | + | + | + | − | − | +/− |
| apbE | Putative thiamine biosynthesis lipoprotein | + | + | + | + | + | + | + | + | + | + | + |
| blc | Outer membrane lipoprotein (lipocalin) | + | + | + | + | + | + | + | + | + | + | + |
| cutF | Outer membrane lipoprotein | + | + | + | + | + | + | + | + | + | + | + |
| hmsF | Putative lipoprotein | + | + | + | + | + | + | + | + | + | − | + |
| lgt | Phosphatidylglycerol-prolipoprotein diacylglyceryl transferase | + | + | + | + | + | + | + | + | + | + | + |
| lnt | Apolipoprotein N-acyltransferase | + | + | + | + | + | + | + | + | + | + | + |
| lolA | Outer membrane lipoproteins | + | + | + | + | + | + | + | + | + | + | + |
| lpp | Murein lipoprotein | + | + | + | + | + | + | + | + | + | + | +/− |
| nlpB | Lipoprotein 34 | + | + | + | + | + | + | + | + | + | + | + |
| nlpC | Lipoprotein | + | + | + | + | + | + | + | + | + | + | + |
| nlpD | Lipoprotein | + | + | + | + | + | + | + | + | + | + | + |
| nlpI | Lipoprotein | + | + | + | + | + | + | + | + | + | + | + |
| osmB | Lipoprotein, osmotically inducible | + | + | + | + | + | + | + | + | + | + | + |
| rlpAB | Minor lipoprotein | + | + | + | + | + | + | + | + | + | + | + |
| slyB | Putative outer membrane lipoprotein | + | + | + | + | + | + | + | + | + | + | + |
| spr | Suppresses thermosensitivity of prc mutants at low osmolarity | + | + | + | + | + | + | + | + | + | + | + |
| vacJ | Lipoprotein precursor | + | + | + | + | + | + | + | + | + | + | + |
| yafL | Putative lipoprotein | + | + | + | + | + | + | + | + | + | + | + |
| yajG | Putative lipoprotein | + | + | +/− | +/− | +/− | + | + | +/− | +/− | +/− | +/− |
| yehR | Putative lipoprotein | + | + | + | + | + | + | + | + | + | + | + |
| yfiO | Putative lipoprotein with tetratricopeptide repeat domain | + | + | + | + | + | + | + | + | + | + | + |
| yqhH | Putative outer membrane lipoprotein | + | + | + | + | + | + | + | + | + | + | + |
| yhiU | Multidrug resistance protein | +/− | + | + | + | + | + | + | +/− | +/− | +/− | +/− |
Only microarray data are given, not PCR confirmation results.
+, present; −, absent; +/−, divergent.
Protease.
Proteases were shown to be present in many pathogenic bacteria, where they play critical functions related to colonization and evasion of host immune defenses or tissue damage during infection (47, 65). Protease functions as an endopeptidase, signal peptidase, or aminopeptidase. Proteolysis in E. coli serves to rid the cell of abnormal and misfolded proteins and to limit the time and amounts of availability of critical regulatory proteins. Most intracellular proteolysis is initiated by energy-dependent proteases, including Lon, ClpXP, and HflB (24). Oligonucleotides which represent 137 proteases were printed on our microarray. Approximately 70% of them (102 of 137) are shown to be present in all 11 representative strains. For the remaining proteases, some ORFs exhibit nonrandom distribution among representative strains. Of interest, vat.2, encoding hemoglobin protease (55), is present in all strains in group 1 but absent from group 2 strains (Table 5). Similarly, yeaZ, encoding a putative glycoprotein endopeptidase, is absent only in group 2 strains EC10 and RS168 (Table 5). The gene sohA, encoding a putative protease, allows temperature-sensitive htrA mutant E. coli to grow at 42°C (4). The microarray showed that sohA is present in all group 2 strains and one group 1 strain, RS167 (Table 5).
TABLE 5.
Distribution of proteases among meningitis-causing E. coli K1 strainsa
| ORF | Function(s) | Presence of ORF in strainb:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RS218 | C5 | IHE3034 | S88 | S95 | A90 | RS167 | E334 | EC10 | RS168 | E253 | ||
| ampE | Putative transmembrane protein, putative protease | + | + | + | + | + | + | + | + | + | + | + |
| clpA | Serine protease | + | + | + | + | + | + | + | + | + | + | + |
| degQ | Serine endoprotease | + | + | + | + | + | + | + | + | + | + | + |
| degS | Periplasmic serine endoprotease | + | + | + | + | + | + | + | + | + | + | + |
| ecfE | Membrane-associated protease | + | + | + | + | + | + | + | + | + | + | + |
| envC | Periplasmic protease | + | + | + | + | + | + | + | + | + | + | + |
| gcp | Putative O-sialoglycoprotein endopeptidase | + | + | + | + | + | + | + | + | + | + | + |
| hflB | ATP-dependent zinc metalloprotease | + | + | + | + | + | + | + | + | + | + | + |
| hslU | ATPase component of the HslUV protease | + | + | + | + | + | + | + | + | + | + | + |
| htrA | Periplasmic serine protease | + | + | + | + | + | + | + | + | + | + | + |
| hycI | Protease involved in processing C-terminal end of HycE | + | + | + | + | + | + | + | + | + | + | + |
| iap | Aminopeptidase in alkaline phosphatase isozyme conversion | + | + | + | + | + | + | + | + | + | + | + |
| lepB | Signal peptidase I | +/− | +/− | +/− | +/− | +/− | − | − | +/− | +/− | +/− | − |
| lexA | Transcriptional repressor for SOS response (signal peptidase of LexA family) | + | + | + | + | + | + | + | + | + | + | + |
| lon | DNA-binding ATP-dependent protease La | + | + | + | + | + | + | + | + | − | − | + |
| lspA | Prolipoprotein signal peptidase (SPase II) | + | + | + | + | + | + | + | + | + | + | + |
| map | Methionine aminopeptidase | + | + | + | + | + | + | + | + | + | + | + |
| mepA | Murein dd-endopeptidase | + | + | + | + | + | + | + | + | + | + | + |
| ompX | Outer membrane protease, receptor for phage OX2 | + | + | + | + | + | + | + | + | + | + | + |
| pbpG | d-Alanyl-d-alanine endopeptidase | + | + | + | + | + | + | + | + | + | + | + |
| pepA | Aminopeptidase A | + | + | + | + | + | + | + | + | + | + | + |
| prc | Carboxy-terminal protease | + | + | + | +/− | + | + | + | + | + | + | + |
| ptrA | Protease III | + | + | + | + | + | + | + | + | + | + | + |
| ptrB | Protease II | + | + | + | + | + | + | + | + | + | + | + |
| radA | Putative ATP-dependent protease | + | + | + | + | + | + | + | + | + | + | + |
| recA | DNA strand-exchange and recombination protein with protease and nuclease activity | + | + | + | + | + | + | + | + | − | + | + |
| rzpR | Putative prophage lambda endopeptidase | + | + | + | + | + | + | + | + | + | + | + |
| sohA | Putative protease; htrA suppressor protein | − | − | − | − | − | − | + | − | + | + | + |
| sppA | Protease IV, a signal peptide peptidase | + | +/− | +/− | +/− | +/− | +/− | +/− | +/− | + | + | +/− |
| sspB | Stringent starvation protein B | + | + | + | + | + | + | + | + | + | + | + |
| tesA | Protease I | + | + | + | + | + | + | + | + | + | + | + |
| vat.2 | Hemoglobin protease | + | + | + | + | + | + | + | + | − | − | − |
| yeaZ | Putative glycoprotein endopeptidase | + | + | + | + | + | + | + | + | − | − | + |
| yegQ | Putative protease | + | + | + | + | + | + | + | + | + | + | + |
| yghQ | Putative serine protease | + | + | + | + | + | + | + | + | + | + | + |
| yhbU | Putative protease | + | + | + | + | + | + | + | + | + | + | + |
| yhbV | Putative protease | + | + | + | + | + | + | + | + | + | + | + |
Only microarray data are given, not PCR confirmation results.
+, present; −, absent; +/−, divergent.
Outer membrane protein.
There is increasing evidence that outer membrane proteins contribute to adhesion and invasion of the host cells in several gram-negative organisms. For example, outer membrane protein A (OmpA), a highly conserved protein in E. coli, was shown to contribute to E. coli K1 association with HBMEC and penetration into the central nervous system (66). One hundred forty-three oligonucleotides which represent outer membrane proteins and outer membrane protein-related proteins were printed onto our microarray. Microarray data showed that many outer membrane proteins were absent in group 1 (strains RS218, IHE3034, C5, RS167, A90, S88, S95, and E334) but present in group 2 (strains EC10, E253, and RS168) (Table 6). For example, ycbS, encoding a putative outer membrane protein; yejO, encoding a putative outer membrane protein with a pectin lyase-like domain; and yiaT, encoding a putative outer membrane protein, are present in all group 2 strains but absent in group 1 strains. Similarly, sfmD, encoding a putative outer membrane protein, is present in all group 2 strains but absent in group 1 strains. In contrast, some other outer membrane proteins are present only in group 1 strains. For example, bglH, encoding a carbohydrate-specific outer membrane porin, is one gene of the bgl operon, which is responsible for uptake and fermentation of β-glucosides in E. coli (1). Our results showed that bglH is present in all group 1 strains but absent in group 2 strains except for strain E253 (Table 6).
TABLE 6.
Distribution of outer membrane proteins among meningitis-causing E. coli K1 strainsa
| ORF | Function | Presence of ORF in strainb:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RS218 | C5 | IHE3034 | S88 | S95 | A90 | RS167 | E334 | EC10 | RS168 | E253 | ||
| bglH | Carbohydrate-specific outer membrane porin in cryptic operon | + | + | + | + | + | + | + | + | − | − | + |
| btuB | Cobalamin transport, receptor for E colicins | + | + | + | + | + | + | + | + | + | + | + |
| cirA | Receptor for colicin I | + | + | + | + | + | + | + | + | + | + | + |
| eaeH | Putative outer membrane protein important for attachment to cell | + | +/− | +/− | +/− | +/− | +/− | +/− | +/− | + | + | + |
| fadL | Transport of long-chain fatty acids | + | + | + | + | + | + | + | + | + | + | + |
| fecA | Receptor for ferric citrate | − | − | − | + | + | + | − | − | + | + | + |
| fepA | Receptor for ferric enterobactin and colicins B and D | + | + | + | + | + | + | + | + | + | + | + |
| hmsH | Putative outer membrane protein | + | + | + | + | + | + | + | + | + | − | + |
| nfrA | Bacteriophage N4 receptor | +/− | − | − | − | − | − | + | + | + | + | + |
| nmpC | NmpC precursor | + | + | + | + | + | + | + | + | + | + | + |
| ompA | Outer membrane protein 3a | + | + | + | + | + | + | + | + | + | + | + |
| ompC | Outer membrane pore protein 1b | + | + | + | + | + | + | + | + | + | + | + |
| ompF | Outer membrane pore protein 1a | + | + | + | + | + | + | + | + | + | + | + |
| ompG | Outer membrane pore protein | + | + | + | + | + | + | + | + | + | + | + |
| ompN | Outer membrane pore protein N | + | + | + | + | + | + | + | + | + | + | + |
| ompT | Protease VII, outer membrane protein 3b (a), putative porin | + | + | + | + | + | + | + | + | + | + | − |
| ompW | Colicin S4 receptor | + | + | + | + | + | + | + | + | + | + | + |
| pldA | Outer membrane phospholipase A | + | + | + | + | + | + | + | + | + | + | + |
| sfmD | Putative outer membrane protein | − | − | − | − | − | − | − | − | + | + | + |
| slp | Outer membrane protein, induced after carbon starvation | + | +/− | + | + | + | + | + | +/− | + | + | + |
| yaeT | Putative outer membrane antigen | +/− | +/− | +/− | +/− | +/− | +/− | +/− | + | +/− | +/− | + |
| yaiV | Putative outer membrane protein with cyclic AMP-binding domain | + | + | + | + | + | + | + | + | + | + | + |
| ybiL | Putative ferrisiderophore receptor | + | + | + | + | + | + | + | + | + | + | + |
| ycbS | Putative outer membrane protein | − | − | − | − | − | − | − | − | + | + | + |
| ydeT | Putative outer membrane protein | − | + | + | − | + | + | + | +/− | + | + | − |
| yehB | Putative outer membrane protein | + | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | + |
| yejO | Putative outer membrane protein with pectin lyase-like domain | − | − | − | − | − | − | − | +/− | + | + | + |
| yfiB | Putative outer membrane protein | + | + | + | + | + | + | + | + | + | + | + |
| yiaD | Putative outer membrane protein | + | + | + | + | + | + | + | + | + | + | + |
| yiaT | Putative outer membrane protein | − | − | − | − | − | − | − | +/− | + | + | + |
| yjcP | Putative outer membrane protein | + | + | + | + | + | + | + | + | + | + | + |
| yjhA | Conserved hypothetical protein, outer membrane domain | + | + | + | + | + | + | + | + | + | + | + |
| yjiK | Putative outer membrane protein | + | + | + | +/− | +/− | + | + | + | + | + | + |
| yncD | Putative outer membrane porin protein | + | + | + | + | + | + | + | + | + | + | + |
| yohG | Putative outer membrane protein | + | + | + | + | + | + | + | + | + | + | + |
| ypjA | Putative outer membrane protein with pectin lyase-like domain | + | + | + | + | + | + | + | + | + | + | + |
| ytfM | Putative outer membrane protein | + | + | + | + | + | + | + | + | + | + | + |
Only microarray data are given, not PCR confirmation results.
+, present; −, absent; +/−, divergent.
Secretion system.
General secretory pathway (GSP) systems, which can export the majority of bacterial exoenzymes and toxins, have been identified in many gram-negative bacteria, such as Haemophilus influenzae, V. cholerae, uropathogenic E. coli, and Helicobacter pylori (56, 60). Secretion by the GSP has been shown to play an important role in bacterial pathogenesis. For example, many virulence factors including extracellular toxins, pili, curli, adhesins, invasins, and proteases are exported to the extracellular environment via the GSP (62). Our microarray data showed the presence of the GSP operon (13 ORFs) in all group 1 strains but not in group 2 strains except for strain E253 (Table 7).
TABLE 7.
Distribution of type III secretion system and GSP genes among meningitis-causing E. coli K1 strains
| Gene | Presence of gene in straina:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RS218 | C5 | IHE3034 | S88 | S95 | A90 | RS167 | E334 | EC10 | RS168 | E253 | |
| sepb | − | − | − | − | − | − | − | − | − | − | − |
| escb | − | − | − | − | − | − | − | − | − | − | − |
| eprb | − | − | − | − | − | − | − | − | + | + | + |
| epab | − | − | − | − | − | − | − | − | + | + | + |
| eivb | − | − | − | − | − | − | − | − | + | − | − |
| GSPc,d | + | + | + | + | + | + | + | + | − | − | + |
+, present; −, absent.
PCR confirmation results.
Including all 13 ORFs of the GSP operon.
Only microarray data are given, not PCR confirmation results.
Type III secretion systems (TTSSs) allow Yersinia spp., Salmonella spp., Shigella spp., Pseudomonas aeruginosa, and enteropathogenic E. coli to adhere to the surface of eukaryotic cells by injecting bacterial proteins across the two bacterial membranes and the eukaryotic cell membrane to destroy or subvert the target cell (12, 45). These systems consist of a secretion apparatus, made of approximately 25 proteins, and an array of proteins released by this apparatus. Some of these released proteins are “effectors,” which are delivered into the cytosol of the target cell, whereas the others are “translocators,” which help the effectors cross the membrane of the eukaryotic cell. Most of the effectors act on the cytoskeleton or on intracellular signaling cascades (13). A protein injected by the enteropathogenic E. coli serves as a membrane receptor for the docking of the bacterium itself at the surface of the cell (23). Interestingly, by using comparative genomic hybridization, we found that all group 2 strains harbor the type III secretion system locus (Table 7). This locus contains ORFs whose amino acid sequences show high degrees of similarity with those of the proteins that make up the type III secretion apparatus of the inv-spa-prg locus on a Salmonella SPI-1 pathogenicity island (61). This locus was designated ETT2 (E. coli type III secretion 2) and consisted of the epr, epa, and eiv genes. ETT2 was found in enteropathogenic E. coli and some non-O157 Shiga toxin-producing E. coli strains, but most of them contained a truncated portion of ETT2 (30). Strains RS168 and E253 harbor a part of ETT2 (epr and epa operon) but lack eiv genes. However, strain EC10 was found to harbor all the genes needed to encode type III secretion apparatus proteins. This is the first demonstration that the type III secretion system is found to be present in E. coli K1 strains isolated from CSF.
DISCUSSION
E. coli is the most common gram-negative organism that causes meningitis during the neonatal period and presents a major burden for the public health system. Further improvement in the treatment of neonatal meningitis will require new therapeutic approaches and a complete understanding of the underlying pathogenic mechanisms. Several microbial factors such as the K1 capsule, OmpA, type I fimbriae, Ibe proteins, AslA, and CNF1 were shown to be involved in E. coli meningitis and have been characterized (3, 32, 34, 40, 64, 66). However, most of the meningitis-causing E. coli K1 isolates do not harbor all of those known microbial determinants. For example, we have previously reported that CNF1 is a virulence factor contributing to E. coli K1 invasion of HBMEC in vitro and traversal of the BBB in vivo (40), but cnf1 was found to be present in only two (strains C5 and RS218) of 11 representative meningitis-causing E. coli K1 strains (Fig. 3B). Thus, it is likely that the remaining strains utilize other microbial determinants to invade HBMEC and traverse the BBB. At present, the distribution of potential microbial virulence factors in meningitis-causing E. coli K1 strains is poorly understood.
It has been known that some virulence factors can be encoded by mobile genetic islands, which are capable of lateral gene transfer. Besides the core genetic contents to maintain primary metabolism, some strains may acquire different extra genetic elements which can help them adapt to specific niches. Meningitis-causing E. coli K1 strains have been shown to obtain some strain-specific genetic elements (33). To understand the roles of these genetic elements in the pathogenesis of meningitis, determining the distribution of known and putative virulence factors should be an important first step. CGH is a powerful tool for this purpose. For CGH, the more oligonucleotide probes that are printed on the slide, the more data that can be obtained. We constructed a comprehensive E. coli microarray harboring 8,144 ORFs. CGH data showed that the distribution of known and putative virulence factors among 11 representative strains is not random. Some of them are present in particular strains and absent in other strains or vice versa. These findings provided novel insights into the evolution and relationship of meningitis-associated E. coli K1 strains and suggested potential targets for prevention and therapy of E. coli meningitis.
Clustering data revealed that these 11 representative meningitis-causing E. coli K1 strains can be divided into two different groups (Fig. 2). Strains RS218, IHE3034, C5, A90, RS167, E334, S88, and S95 are grouped together and named group 1. The other group includes strains EC10, RS168, and E253 and is named group 2. This finding based on our comprehensive microarray analysis revealed that E. coli K1 strains with different O serotypes may belong to the same group (e.g., O18, O1, O16, O12, and O45 for group 1 versus O1, O2, and O12 for group 2), while E. coli K1 strains with the same O serotype may belong to different groups (e.g., O1 and O12). We also carried out triplex PCR to determine the phylogenetic relationship of these 11 representative meningitis-causing E. coli K1 strains (11). Results showed that strains RS218, IHE3034, C5, A90, RS167, E334, S88, and S95 belong to group B2 (73%) while strains EC10 and RS168 are in group D (18%) and strain E253 is in group A (9%). The proportions of each phylogenetic group in our E. coli strains matched those of the previous study (7), indicating that our E. coli K1 strains used in this study are indeed representative of the meningitis-causing E. coli K1 strains. Moreover, there is a similarity between our genome-based grouping and the previously defined phylogenetic grouping. For example, phylogenetic group B2, which is predominant in CSF isolates of E. coli K1, belongs to our group 1, while less common phylogenetic groups A and D belong to our group 2.
Most remarkably, we showed that 3 of the 11 representative strains harbor some genes from ETT2 (Table 7) and these three strains belong to group 2, while none of the group 1 strains harbor ETT2. The function and mechanism of the TTSS have been well documented in Yersinia spp., Salmonella spp., Shigella spp., and P. aeruginosa (12, 45). The TTSS is found exclusively among gram-negative bacteria and is responsible for the transport of proteins across the inner bacterial membrane, the peptidoglycan layer, and the outer bacterial membrane, as well as across host cell barriers such as the plasma membrane and in some instances such as the plant cell wall, into the host cell interior (12, 45). The TTSS apparatus used to deliver these effectors is conserved and shows functional complementarity for secretion and translocation. ETT2 was also found in EPEC and some non-O157 Shiga toxin-producing E. coli strains, but most of them contained a truncated portion of ETT2 (30).
The existence of a degenerate ETT2 gene cluster was found in septicemic E. coli strain 789 (36). Sequence analysis of the ETT2 genes showed premature stop codons in eprI and eprJ, encoding the needle structure, and deletion of the invG gene, which encodes a conserved component of the outer membrane ring. This ETT2 lacks the gene (eivC) for the cytoplasmic ATPase that energizes secretion and some other conserved components of the TTSS (e.g., epaS). However, a deletion mutant of genes coding for the putative inner membrane ring of the secretion complex showed significantly reduced virulence in a 1-day-old chick model, even though the mutation does not seem to affect the secreted proteome (36). Of interest, strain EC10 from group 2, which was isolated from the CSF of a neonate with meningitis, was found to harbor all the genes needed to encode type III secretion apparatus proteins compared to the above-mentioned septicemic E. coli strain 789. We speculate that strain EC10 may utilize the TTSS to invade and subvert the signal transduction pathway in HBMEC to induce meningitis. However, our microarray data failed to reveal the presence of effectors and translocators in group 2 strains such as sep, esc, and tir which were identified in the locus for enterocyte effacement (LEE) (48). Thus, group 2 strains may employ some other effectors and translocators to utilize the TTSS. For example, effectors which are non-LEE-encoded type III translocated virulence factors have been identified in EHEC (27). Studies are in progress to determine the potential contribution of ETT2 to the pathogenesis of E. coli meningitis caused by strain EC10.
The general secretory pathway is used by many gram-negative bacteria to transport exoproteins from the periplasm to the outside milieu (56). We showed that all group 1 strains harbor the general secretory pathway system, but group 2 strains do not have such a system, except for strain E253 (Table 7). It is tempting to speculate that group 1 strains utilize the general secretory pathway system during infection. We searched all the sequenced E. coli genomes and found that uropathogenic E. coli strain CFT073 also harbors the GSP system instead of the TTSS. However, EHEC strains DEL933 and Sakai (O157) do not have GSP systems and harbor the typical TTSS. We speculate that either GSP or TTSS may have been maintained during evolution because of their similar function. Additional studies are needed to clarify this speculation.
In conclusion, we carried out comparative genomic hybridization in representative strains of meningitis-causing E. coli K1 and provide evidence that these E. coli K1 strains can be divided into two groups. Based on the distribution of putative virulence factors, lipoproteins, proteases, outer membrane proteins, and secretion systems, we speculate that these two groups of E. coli K1 strains may cause meningitis by using different mechanisms. This speculation is also supported by our demonstration of the TTSS mainly in group 2 strains of E. coli K1. Additional studies with a larger collection of E. coli K1 strains isolated from CSF are needed to determine whether our proposed grouping can be applied to all the CSF isolates of E. coli K1.
Supplementary Material
Acknowledgments
We thank Edouard Bingen and Timo Korhonen for providing E. coli strains.
This work was supported by NIH grants R01-NS 026310 and AI47225.
Editor: F. C. Fang
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Andersen, C., B. Rak, and R. Benz. 1999. The gene bglH present in the bgl operon of Escherichia coli, responsible for uptake and fermentation of beta-glucosides, encodes for a carbohydrate-specific outer membrane porin. Mol. Microbiol. 31:499-510. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, and R. E. Kingston (ed.). 1992. Short protocols in molecular biology, 2nd ed. Wiley, New York, N.Y.
- 3.Badger, J. L., C. A. Wass, S. J. Weissman, and K. S. Kim. 2000. Application of signature-tagged mutagenesis for identification of Escherichia coli K1 genes that contribute to invasion of human brain microvascular endothelial cells. Infect. Immun. 68:5056-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baird, L., and C. Georgopoulos. 1990. Identification, cloning, and characterization of the Escherichia coli sohA gene, a suppressor of the htrA (degP) null phenotype. J. Bacteriol. 172:1587-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnich, N., M. A. Bringer, L. Claret, and A. Darfeuille-Michaud. 2004. Involvement of lipoprotein NlpI in the virulence of adherent invasive Escherichia coli strain LF82 isolated from a patient with Crohn's disease. Infect. Immun. 72:2484-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass, S., Q. Gu, and A. Christen. 1996. Multicopy suppressors of prc mutant Escherichia coli include two HtrA (DegP) protease homologs (HhoAB), DksA, and a truncated R1pA. J. Bacteriol. 178:1154-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bingen, E., B. Picard, N. Brahimi, S. Mathy, P. Desjardins, J. Elion, and E. Denamur. 1998. Phylogenetic analysis of Escherichia coli strains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J. Infect. Dis. 177:642-650. [DOI] [PubMed] [Google Scholar]
- 8.Bonacorsi, S., O. Clermont, V. Houdouin, C. Cordevant, N. Brahimi, A. Marecat, C. Tinsley, X. Nassif, M. Lange, and E. Bingen. 2003. Molecular analysis and experimental virulence of French and North American Escherichia coli neonatal meningitis isolates: identification of a new virulent clone. J. Infect. Dis. 187:1895-1906. [DOI] [PubMed] [Google Scholar]
- 9.Boquet, P. 2001. The cytotoxic necrotizing factor 1 (CNF1) from Escherichia coli. Toxicon 39:1673-1680. [DOI] [PubMed] [Google Scholar]
- 10.Braun, V., and H. C. Wu. 1993. Lipoproteins, structure, function, biosynthesis and model for protein export. New Compr. Biochem. 27:319-342. [Google Scholar]
- 11.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 14.Cross, A. S., K. S. Kim, D. C. Wright, J. C. Sadoff, and P. Gemski. 1986. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic strains of Escherichia coli. J. Infect. Dis. 154:497-503. [DOI] [PubMed] [Google Scholar]
- 15.Dawson, K. G., J. C. Emerson, and J. L. Burns. 1999. Fifteen years of experience with bacterial meningitis. Pediatr. Infect. Dis. J. 18:816-822. [DOI] [PubMed] [Google Scholar]
- 16.de Lorenzo, V., A. Bindereif, B. H. Paw, and J. B. Neilands. 1986. Aerobactin biosynthesis and transport genes of plasmid ColV-K30 in Escherichia coli K-12. J. Bacteriol. 165:570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lorenzo, V., and J. B. Neilands. 1986. Characterization of iucA and iucC genes of the aerobactin system of plasmid ColV-K30 in Escherichia coli. J. Bacteriol. 167:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dziejman, M., E. Balon, D. Boyd, C. M. Fraser, J. F. Heidelberg, and J. J. Mekalanos. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc. Natl. Acad. Sci. USA 99:1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewers, C., T. Janssen, S. Kiessling, H. C. Philipp, and L. H. Wieler. 2004. Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from colisepticemia in poultry. Vet. Microbiol. 104:91-101. [DOI] [PubMed] [Google Scholar]
- 21.Feria, C. P., J. D. Correia, J. Goncalves, and J. Machado. 2000. Detection of virulence factors in uropathogenic Escherichia coli isolated from humans, dogs and cats in Portugal. Adv. Exp. Med. Biol. 485:305-308. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg, M. B., S. A. Boyko, J. R. Butterton, J. A. Stoebner, S. M. Payne, and S. B. Calderwood. 1992. Characterization of a Vibrio cholerae virulence factor homologous to the family of TonB-dependent proteins. Mol. Microbiol. 6:2407-2418. [DOI] [PubMed] [Google Scholar]
- 23.Goosney, D. L., S. Gruenheid, and B. B. Finlay. 2000. Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu. Rev. Cell Dev. Biol. 16:173-189. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 30:465-506. [DOI] [PubMed] [Google Scholar]
- 25.Grimwood, K., P. Anderson, V. Anderson, L. Tan, and T. Nolan. 2000. Twelve year outcomes following bacterial meningitis: further evidence for persisting effects. Arch. Dis. Child. 83:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross, R. J., L. R. Ward, E. J. Threlfall, T. Cheasty, and B. Rowe. 1983. Drug resistance among Escherichia coli strains isolated from cerebrospinal fluid. J. Hyg. (London) 90:195-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruenheid, S., I. Sekirov, N. A. Thomas, W. Deng, P. O'Donnell, D. Goode, Y. Li, E. A. Frey, N. F. Brown, P. Metalnikov, T. Pawson, K. Ashman, and B. B. Finlay. 2004. Identification and characterization of NleA, a non-LEE-encoded type III translocated virulence factor of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 51:1233-1249. [DOI] [PubMed] [Google Scholar]
- 28.Gupta, S. D., B. T. Lee, J. Camakaris, and H. C. Wu. 1995. Identification of cutC and cutF (nlpE) genes involved in copper tolerance in Escherichia coli. J. Bacteriol. 177:4207-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara, H., Y. Yamamoto, A. Higashitani, H. Suzuki, and Y. Nishimura. 1991. Cloning, mapping, and characterization of the Escherichia coli prc gene, which is involved in C-terminal processing of penicillin-binding protein 3. J. Bacteriol. 173:4799-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartleib, S., R. Prager, I. Hedenstrom, S. Lofdahl, and H. Tschape. 2003. Prevalence of the new, SPI1-like, pathogenicity island ETT2 among Escherichia coli. Int. J. Med. Microbiol. 292:487-493. [DOI] [PubMed] [Google Scholar]
- 31.Hinchliffe, S. J., K. E. Isherwood, R. A. Stabler, M. B. Prentice, A. Rakin, R. A. Nichols, P. C. Oyston, J. Hinds, R. W. Titball, and B. W. Wren. 2003. Application of DNA microarrays to study the evolutionary genomics of Yersinia pestis and Yersinia pseudotuberculosis. Genome Res. 13:2018-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman, J. A., J. L. Badger, Y. Zhang, S. H. Huang, and K. S. Kim. 2000. Escherichia coli K1 aslA contributes to invasion of brain microvascular endothelial cells in vitro and in vivo. Infect. Immun. 68:5062-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houdouin, V., S. Bonacorsi, N. Brahimi, O. Clermont, X. Nassif, and E. Bingen. 2002. A uropathogenicity island contributes to the pathogenicity of Escherichia coli strains that cause neonatal meningitis. Infect. Immun. 70:5865-5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang, S. H., Y. H. Chen, G. Kong, S. H. Chen, J. Besemer, M. Borodovsky, and A. Jong. 2001. A novel genetic island of meningitic Escherichia coli K1 containing the ibeA invasion gene (GimA): functional annotation and carbon-source-regulated invasion of human brain microvascular endothelial cells. Funct. Integr. Genomics 1:312-322. [DOI] [PubMed] [Google Scholar]
- 35.Huang, S. H., Z. S. Wan, Y. H. Chen, A. Y. Jong, and K. S. Kim. 2001. Further characterization of Escherichia coli brain microvascular endothelial cell invasion gene ibeA by deletion, complementation, and protein expression. J. Infect. Dis. 183:1071-1078. [DOI] [PubMed] [Google Scholar]
- 36.Ideses, D., U. Gophna, Y. Paitan, R. R. Chaudhuri, M. J. Pallen, and E. Z. Ron. 2005. A degenerate type III secretion system from septicemic Escherichia coli contributes to pathogenesis. J. Bacteriol. 187:8164-8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janben, T., C. Schwarz, P. Preikschat, M. Voss, H. C. Philipp, and L. H. Wieler. 2001. Virulence-associated genes in avian pathogenic Escherichia coli (APEC) isolated from internal organs of poultry having died from colibacillosis. Int. J. Med. Microbiol. 291:371-378. [DOI] [PubMed] [Google Scholar]
- 38.Johnson, J. R., S. Jelacic, L. M. Schoening, C. Clabots, N. Shaikh, H. L. Mobley, and P. I. Tarr. 2005. The IrgA homologue adhesin Iha is an Escherichia coli virulence factor in murine urinary tract infection. Infect. Immun. 73:965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson, J. R., E. Oswald, T. T. O'Bryan, M. A. Kuskowski, and L. Spanjaard. 2002. Phylogenetic distribution of virulence-associated genes among Escherichia coli isolates associated with neonatal bacterial meningitis in the Netherlands. J. Infect. Dis. 185:774-784. [DOI] [PubMed] [Google Scholar]
- 40.Khan, N. A., Y. Wang, K. J. Kim, J. W. Chung, C. A. Wass, and K. S. Kim. 2002. Cytotoxic necrotizing factor-1 contributes to Escherichia coli K1 invasion of the central nervous system. J. Biol. Chem. 277:15607-15612. [DOI] [PubMed] [Google Scholar]
- 41.Kim, K. S., and B. F. Anthony. 1983. Efficacy of trimethoprim/sulfamethoxazole in experimental Escherichia coli bacteremia and meningitis. Chemotherapy 29:428-435. [DOI] [PubMed] [Google Scholar]
- 42.Kim, K. S., J. H. Kang, and A. S. Cross. 1986. The role of capsular antigens in serum resistance and in vivo virulence of Escherichia coli. FEMS Microbiol. Lett. 35:275-278. [Google Scholar]
- 43.Korczak, B., J. Frey, J. Schrenzel, G. Pluschke, R. Pfister, R. Ehricht, and P. Kuhnert. 2005. Use of diagnostic microarrays for determination of virulence gene patterns of Escherichia coli K1, a major cause of neonatal meningitis. J. Clin. Microbiol. 43:1024-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korhonen, T. K., M. V. Valtonen, J. Parkkinen, V. Vaisanen-Rhen, J. Finne, F. Orskov, I. Orskov, S. B. Svenson, and P. H. Makela. 1985. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect. Immun. 48:486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee, C. A. 1997. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 5:148-156. [DOI] [PubMed] [Google Scholar]
- 46.Madan Babu, M., and K. Sankaran. 2002. DOLOP—database of bacterial lipoproteins. Bioinformatics 18:641-643. [DOI] [PubMed] [Google Scholar]
- 47.Maeda, H. 1996. Role of microbial proteases in pathogenesis. Microbiol. Immunol. 40:685-699. [DOI] [PubMed] [Google Scholar]
- 48.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23:399-407. [DOI] [PubMed] [Google Scholar]
- 49.Meier, C., T. A. Oelschlaeger, H. Merkert, T. K. Korhonen, and J. Hacker. 1996. Ability of Escherichia coli isolates that cause meningitis in newborns to invade epithelial and endothelial cells. Infect. Immun. 64:2391-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mundy, R., C. Jenkins, J. Yu, H. Smith, and G. Frankel. 2004. Distribution of espI among clinical enterohaemorrhagic and enteropathogenic Escherichia coli isolates. J. Med. Microbiol. 53:1145-1149. [DOI] [PubMed] [Google Scholar]
- 51.Narita, S., K. Tanaka, S. Matsuyama, and H. Tokuda. 2002. Disruption of lolCDE, encoding an ATP-binding cassette transporter, is lethal for Escherichia coli and prevents release of lipoproteins from the inner membrane. J. Bacteriol. 184:1417-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohara, M., H. C. Wu, K. Sankaran, and P. D. Rick. 1999. Identification and characterization of a new lipoprotein, NlpI, in Escherichia coli K-12. J. Bacteriol. 181:4318-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okeke, I. N., I. C. Scaletsky, E. H. Soars, L. R. Macfarlane, and A. G. Torres. 2004. Molecular epidemiology of the iron utilization genes of enteroaggregative Escherichia coli. J. Clin. Microbiol. 42:36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 55.Pamnani, V., T. Tamura, A. Lupas, J. Peters, Z. Cejka, W. Ashraf, and W. Baumeister. 1997. Cloning, sequencing and expression of VAT, a CDC48/p97 ATPase homologue from the archaeon Thermoplasma acidophilum. FEBS Lett. 404:263-268. [DOI] [PubMed] [Google Scholar]
- 56.Pugsley, A. P., O. Francetic, O. M. Possot, N. Sauvonnet, and K. R. Hardie. 1997. Recent progress and future directions in studies of the main terminal branch of the general secretory pathway in Gram-negative bacteria—a review. Gene 192:13-19. [DOI] [PubMed] [Google Scholar]
- 57.Rippere-Lampe, K. E., A. D. O'Brien, R. Conran, and H. A. Lockman. 2001. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf1) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immun. 69:3954-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robbins, J. B., G. H. McCracken, Jr., E. C. Gotschlich, F. Orskov, I. Orskov, and L. A. Hanson. 1974. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N. Engl. J. Med. 290:1216-1220. [DOI] [PubMed] [Google Scholar]
- 59.Salama, N., K. Guillemin, T. K. McDaniel, G. Sherlock, L. Tompkins, and S. Falkow. 2000. A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc. Natl. Acad. Sci. USA 97:14668-14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandkvist, M., V. Morales, and M. Bagdasarian. 1993. A protein required for secretion of cholera toxin through the outer membrane of Vibrio cholerae. Gene 123:81-86. [DOI] [PubMed] [Google Scholar]
- 61.Shea, J. E., M. Hensel, C. Gleeson, and D. W. Holden. 1996. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stathopoulos, C., D. R. Hendrixson, D. G. Thanassi, S. J. Hultgren, J. W. St. Geme III, and R. Curtiss III. 2000. Secretion of virulence determinants by the general secretory pathway in gram-negative pathogens: an evolving story. Microbes Infect. 2:1061-1072. [DOI] [PubMed] [Google Scholar]
- 63.Takase, I., F. Ishino, M. Wachi, H. Kamata, M. Doi, S. Asoh, H. Matsuzawa, T. Ohta, and M. Matsuhashi. 1987. Genes encoding two lipoproteins in the leuS-dacA region of the Escherichia coli chromosome. J. Bacteriol. 169:5692-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teng, C. H., M. Cai, S. Shin, Y. Xie, K. J. Kim, N. A. Khan, F. Di Cello, and K. S. Kim. 2005. Escherichia coli K1 RS218 interacts with human brain microvascular endothelial cells via type 1 fimbria bacteria in the fimbriated state. Infect. Immun. 73:2923-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Travis, J., and J. Potempa. 2000. Bacterial proteinases as targets for the development of second-generation antibiotics. Biochim. Biophys. Acta 1477:35-50. [DOI] [PubMed] [Google Scholar]
- 66.Wang, Y., and K. S. Kim. 2002. Role of OmpA and IbeB in Escherichia coli K1 invasion of brain microvascular endothelial cells in vitro and in vivo. Pediatr. Res. 51:559-563. [DOI] [PubMed] [Google Scholar]
- 67.Wu, H. C. 1996. Biosynthesis of lipoproteins, p. 1005-1014. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 68.Yao, Y., D. E. Sturdevant, A. Villaruz, L. Xu, Q. Gao, and M. Otto. 2005. Factors characterizing Staphylococcus epidermidis invasiveness determined by comparative genomics. Infect. Immun. 73:1856-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yokota, N., T. Kuroda, S. Matsuyama, and H. Tokuda. 1999. Characterization of the LolA-LolB system as the general lipoprotein localization mechanism of Escherichia coli. J. Biol. Chem. 274:30995-30999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.