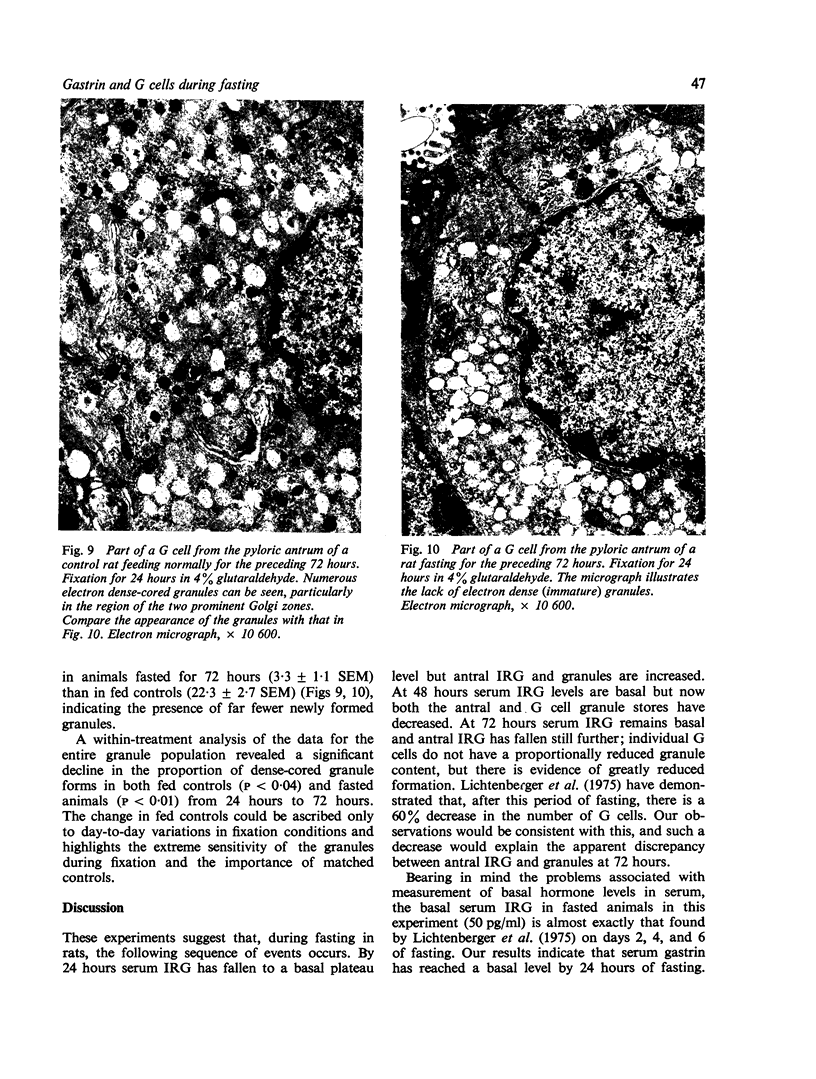
Abstract
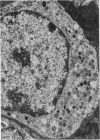
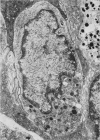
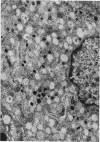
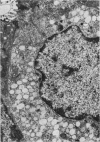
The effect of fasting on serum and antral gastrin concentrations and G cell ultrastructure in the rat has been examined using a radioimmunoassay and quantitative electron microscopy. Serum gastrin levels in fasting animals were markedly reduced and there was also a significant decrease in antral gastrin concentrations after 48 hours and 72 hours of fasting. This was associated with a significant fall in the granule content and cytloplasmic volume of individual G cells, at its greatest by 48 hours. A relative absence of electron dense granules in the Golgi zones of cells from animals fasted for 72 hours suggested a paucity of newly formed granules, but fasting produced no detectable change in the electron density of the granule population taken as a whole. The results indicate that, during fasting, release and then synthesis of gastrin is inhibited, so that granule stores and cell size diminish. The correlation between the granule content of G cells and the antral content of gastrin suggests that hormone release occurs by exocytosis, rather than by any change in the content of individual granules.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G. G. Influence of starvation and refeeding on mucosal size and epithelial renewal in the rat small intestine. Am J Anat. 1972 Apr;133(4):391–400. doi: 10.1002/aja.1001330403. [DOI] [PubMed] [Google Scholar]
- Becker H. D., Reeder D. D., Thompson J. C. Influence of vagotomy on tissue gastrin levels in stomach and pancreas in rats. Surgery. 1973 Nov;74(5):778–782. [PubMed] [Google Scholar]
- Brown H. O., Levine M. L., Lipkin M. Inhibition of intestinal epithelial cell renewal and migration induced by starvation. Am J Physiol. 1963 Nov;205(5):868–872. doi: 10.1152/ajplegacy.1963.205.5.868. [DOI] [PubMed] [Google Scholar]
- Capella C., Solcia E. The endocrine cells of the pig gastrointestinal mucosa and pancreas. Arch Histol Jpn. 1972 Nov;35(1):1–29. doi: 10.1679/aohc1950.35.1. [DOI] [PubMed] [Google Scholar]
- Cowley D. J., Dymock I. W., Boyes B. E., Wilson R. Y., Stagg B. H., Lewin M. R., Polak J. M., Pearse A. G. Zollinger-Ellison syndrome type 1: clinical and pathological correlations in a case. Gut. 1973 Jan;14(1):25–29. doi: 10.1136/gut.14.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt W., Arnold R., Creutzfeldt C., Feurle G., Ketterer H. Gastrin and G-cells in the antral mucosa of patients with pernicious anaemia, acromegaly and hyperparathyroidism and in a Zollinger-Ellison tumour of the pancreas. Eur J Clin Invest. 1971 Sep;1(6):461–479. doi: 10.1111/j.1365-2362.1971.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W., Arnold R., Creutzfeldt C., Track N. S. Mucosal gastrin concentration, molecular forms of gastrin, number and ultrastructure of G-cells in patients with duodenal ulcer. Gut. 1976 Oct;17(10):745–754. doi: 10.1136/gut.17.10.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J., Debas H. T., Walsh J. H., Grossman M. I. Molecular forms of gastrin in antral mucosa and serum of dogs. Proc Soc Exp Biol Med. 1975 Jun;149(2):550–553. doi: 10.3181/00379727-149-38848. [DOI] [PubMed] [Google Scholar]
- Ferreira M. N., Leblond C. P. Argentaffin and other "endocrine" cells of the small intestine in the adult mouse. II. Renewal. Am J Anat. 1971 Jul;131(3):331–339. doi: 10.1002/aja.1001310305. [DOI] [PubMed] [Google Scholar]
- Forssmann W. G., Orci L., Pictet R., Renold A. E., Rouiller C. The endocrine cells in the epithelium of the gastrointestinal mucosa of the rat. An electron microscope study. J Cell Biol. 1969 Mar;40(3):692–715. doi: 10.1083/jcb.40.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssmann W. G., Orci L. Ultrastructure and secretory cycle of the gastrin-producing cell. Z Zellforsch Mikrosk Anat. 1969;101(3):419–432. doi: 10.1007/BF00335578. [DOI] [PubMed] [Google Scholar]
- Ganguli P. C., Polak J. M., Pearse A. G., Elder J. B., Hegarty M. Antral-gastrin-cell hyperplasia in peptic-ulcer disease. Lancet. 1974 Apr 6;1(7858):583–586. doi: 10.1016/s0140-6736(74)92647-6. [DOI] [PubMed] [Google Scholar]
- Johnson L. R., Copeland E. M., Dudrick S. J., Lichtenberger L. M., Castro G. A. Structural and hormonal alterations in the gastrointestinal tract of parenterally fed rats. Gastroenterology. 1975 May;68(5 Pt 1):1177–1183. [PubMed] [Google Scholar]
- Johnson L. R., Lichtenberger L. M., Copeland E. M., Dudrick S. J., Castro G. A. Action of gastrin on gastrointestinal structure and function. Gastroenterology. 1975 May;68(5 Pt 1):1184–1192. [PubMed] [Google Scholar]
- Lehy T., Willems G. Population kinetics of antral gastrin cells in the mouse. Gastroenterology. 1976 Oct;71(4):614–619. [PubMed] [Google Scholar]
- Lichtenberger L. M., Lechago J., Johnson L. R. Depression of antral and serum gastrin concentration by food deprivation in the rat. Gastroenterology. 1975 Jun;68(6):1473–1479. [PubMed] [Google Scholar]
- McGuigan J. E. Immunochemical studies with synthetic human gastrin. Gastroenterology. 1968 Jun;54(6):1005–1011. [PubMed] [Google Scholar]
- McNeill L. K., Hamilton J. R. The effect of fasting on disaccharidase activity in the rat small intestine. Pediatrics. 1971 Jan;47(1):65–72. [PubMed] [Google Scholar]
- Mortensen N. J., Morris J. F. The effect of fixation conditions on the ultrastructural appearance of gastrin cell granules in the rat gastric pyloric antrum. Cell Tissue Res. 1977 Jan 12;176(2):251–263. doi: 10.1007/BF00229466. [DOI] [PubMed] [Google Scholar]
- Odell W. D., Rayford P. L., Ross G. T. Simplified, partially automated method for radioimmunoassay of human thyroid-stimulating, growth, luteinizing, and follicle stimulating hormones. J Lab Clin Med. 1967 Dec;70(6):973–980. [PubMed] [Google Scholar]
- PALADE G. E. A study of fixation for electron microscopy. J Exp Med. 1952 Mar;95(3):285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J. M., Bussolati G., Pearse A. G. Cytochemical, immunofluorescence and ultrastructural investigations on the antral G cells in hyperparathyroidism. Virchows Arch B Cell Pathol. 1971;9(3):187–197. doi: 10.1007/BF02894045. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Doe W., Coulling I., Pearse A. G. The G cells in pernicious anaemia. Gut. 1971 Apr;12(4):319–323. doi: 10.1136/gut.12.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J. M., Hoffbrand A. V., Reed P. I., Bloom S., Pearse A. G. Qualitative and quantitative studies of antral and fundic G cells in pernicious anaemia. Scand J Gastroenterol. 1973;8(4):361–367. [PubMed] [Google Scholar]
- Polak J. M., Stagg B., Pearse A. G. Two types of Zollinger-Ellison syndrome: immunofluorescent, cytochemical and ultrastructural studies of the antral and pancreatic gastrin cells in different clinical states. Gut. 1972 Jul;13(7):501–512. doi: 10.1136/gut.13.7.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayford P. L., Reeder D. D., Thompson J. C. Interlaboratory reproducibility of gastrin measurements by radioimmunoassay. J Lab Clin Med. 1975 Sep;86(3):521–527. [PubMed] [Google Scholar]
- Sato A. Quantitative electron miscroscopic studies on the kinetics of secretory granules in G-cells. Cell Tissue Res. 1978 Feb 14;187(1):45–59. doi: 10.1007/BF00220617. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stadil F., Rehfeld J. F., Christiansen L. A., Malmström Patterns of gastrin components in serum during feeding in normal subjects and duodenal ulcer patients. Scand J Gastroenterol. 1975;10(8):863–868. [PubMed] [Google Scholar]
- Steiner M., Bourges H. R., Freedman L. S., Gray S. J. Effect of starvation on the tissue composition of the small intestine in the rat. Am J Physiol. 1968 Jul;215(1):75–77. doi: 10.1152/ajplegacy.1968.215.1.75. [DOI] [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Studies on the distribution and degradation of heptadecapeptide, big, and big big gastrin. Gastroenterology. 1974 May;66(5):936–943. [PubMed] [Google Scholar]
- Walsh J. H., Grossman M. I. Gastrin (first of two parts). N Engl J Med. 1975 Jun 19;292(25):1324–1334. doi: 10.1056/NEJM197506192922505. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- Willems G., Vansteenkiste Y., Limbosch J. M. Stimulating effect of gastrin on cell proliferation kinetics in canine fundic mucosa. Gastroenterology. 1972 Apr;62(4):583–589. [PubMed] [Google Scholar]
- Willems G., Vansteenkiste Y., Smets P. Effects of food ingestion on the cell proliferation kinetics in the canine fundic mucosa. Gastroenterology. 1971 Sep;61(3):323–327. [PubMed] [Google Scholar]