Abstract
Aim To update conclusions of a previous review of smoking reduction on the extent to which (1) smokers spontaneously reduce their smoking, (2) smokers who try to quit and fail return to smoking less, (3) smokers can substantially reduce and maintain reductions via pharmacological and behavioral treatments and (4) smokers compensate when they reduce.
Method Qualitative systematic review.
Data sources Systematic computer searches and other methods.
Study selection Published and unpublished studies of smokers not trying to stop smoking. We located 13–26 studies for each of the four aims.
Data extraction The first author entered data with confirmation by second author.
Data synthesis Due to the heterogeneity of methods and necessity of extensive recalculation, a meta-analysis was not feasible.
Results Few daily smokers spontaneously reduce. Among those who try to stop smoking and relapse, some return to reduced smoking but whether they maintain this reduction is unclear. Nicotine replacement (and perhaps behavior therapies) can induce smokers not interested in quitting to make significant reductions in their smoking and maintain these over time. Some compensatory smoking occurs with reduction but significant declines in smoke exposure still occur.
Conclusions These results indicate that reduction is feasible when aided by treatment. Whether reduction should be promoted will depend on the effect of reduction on health outcomes and future cessation.
Keywords: Harm reduction, nicotine replacement, smoking, tobacco, tobacco use
INTRODUCTION
The current recommended method to reduce the risk of smoking in smokers is to promote smoking cessation (US Department of Health and Human Services 1990). Efficacy trials indicate population-based interventions can prompt and aid cessation (US Department of Health and Human Services 2000); however, effectiveness is less than optimal (Susser 2002). Efficacy trials also indicate that individually based treatments increase abstinence (Fiore et al. 2000; West, McNeill & Raw 2000); however, again effectiveness is less than optional (Piasecki & Baker 2001; Niaura & Abrams 2002). As a result, in most countries the majority of smokers never quit smoking (Boyle et al. 2000; Gajalakshmi et al. 2000). Even in countries with the most effective cessation interventions—tobacco control policies and cessation aids—less than 2% of smokers stop each year (Giovino 2002).
The above trends have increased interest in several non-cessation methods to reduce tobacco harm (Shiffman et al. 2002). The present paper focuses on one such method, i.e. reducing cigarettes/day (CPD). The utility of the other harm reduction methods has been reviewed elsewhere (Slade & Henningfield 1998; Institute of Medicine 2001; Hatsukami et al. 2002; Shiffman et al. 2002; Warner 2003).
Whether smokers can substantially reduce their CPD and maintain such reduction over time has been debated. The essential feature of drug dependence (including nicotine dependence) is impaired control over drug taking (World Health Organization 1992; American Psychiatric Association 2000; Shadel et al. 2000). Because reducing smoking is a form of controlling drug use, one could argue that, by definition, drug-dependent people should not be able to reduce their smoking. On the other hand, many reviews conclude that reduced consumption is possible for dependent users of non-nicotine drugs (Marlatt & Witkiewitz 2002).
This review updates information from a previous review by the first author (Hughes 2000). Since that review, several reviews of reduced smoking have appeared (Kozlowski et al. 2001; Zellweger 2001; Fager-strom 2002; Jimenez-Ruiz et al. 2002; Tonnesen, in press). However, these reviews were brief and did not include more recent reduction studies. We believed that a more comprehensive and systematic update of the literature would be helpful. The current review focuses on four outcomes: (1) whether smokers reduce their smoking spontaneously; (2) whether smokers who try to quit and fail return to smoking less; (3) whether smokers can substantially reduce and maintain reduction via pharmacological and behavioral treatments; and (4) whether smokers compensate when they reduce. The previous review also focused on whether reduction leads to more or less future abstinence and whether the risks of smoking are decreased; these topics will be covered in a separate paper.
METHODS
Definition of reduced smoking
This paper uses a definition of reduced smoking confined to reducing the number of CPD. We have not included reducing smoking topography (Glasgow, Murray & Lichtenstein 1989) or cigarette length (McMorrow & Fox 1983) because no recent studies have examined these interventions. We have not included reducing tar/nicotine yield because this topic has already been reviewed (National Cancer Institute 2001). We have not included reducing CPD as a method of cessation in smokers actively trying to quit—often termed ‘gradual cessation’ (Cinciripini et al. 1995)—because most harm reduction strategies are focused on those who are not actively trying to quit (Shiffman et al. 2002). Our analysis focuses on clinical studies of adult daily smokers. We have not included short-term human laboratory studies (Ho-Yen et al. 1982; Benowitz et al. 1986) or studies of adolescents (US Department of Health and Human Services 1994; Wetter et al. 2004) pregnant smokers (US Department of Health and Human Services 2001) or smokers with a current psychiatric disorder (Dalack & Meador-Woodruff 1999; Weiner et al. 2001; George et al. 2002).
Data sources
We attempted to follow the Quality of Reporting of Meta-analyses (QUOROM) standards for systematic reviews (Egger, Smith & Altman 2001; Moher et al. 1999). We began with a search of the authors’ own files of articles collected since the last review. We searched Medline, PsychAbstracts and EMBASE databases to December 2004. These databases do not have keywords that correspond well to the topic of smoking reduction, so we searched titles and abstracts for the stems ‘harm reduc-’, ‘smoking reduc-’, ‘cigarette reduc-’, ‘reducing smok-’, ‘reduced smok-’, ‘reduced cig-’, ‘reduction in cig-’ and ‘reducing cig-.’ We used a similar strategy to search the Computer Retrieval of Information on Scientific Projects (CRISP) database of US National Institute of Health grants (www.crisp.cit.nih.gov) and sent a request for publications to the principal investigators of the relevant grants. We examined abstracts of the 2001–04 annual meetings of the Society for Research on Nicotine and Tobacco (SRNT) (www.srnt.org) and the US National and World Conferences on Tobacco or Health (www.nctoh2003.org and www.wctoh2003.org). We also queried the SRNT list-serve (srnt_list@reesgroupinc.com) and relevant pharmaceutical companies for studies. When articles from the above searches cited references that might be relevant, we sought these out. The search ended in July 2004.
Study selection
The only generic inclusion criterion was that the study must have described an attempt at reduction in CPD in adult (≥18 years old) smokers not actively trying to quit smoking. In addition to published and in press articles, we included unpublished papers, abstracts, posters and meeting presentations. Whenever the source was a poster, presentation or abstract, we asked the authors for a full paper to obtain all the possible information about a study. This was usually not successful. We included unpublished studies because most authors of quantitative reviews recommend their inclusion to avoid publication bias (Cook et al. 1993). Many of the studies we review were funded by pharmaceutical companies and publication bias among such companies has been documented (Cook et al. 1993). As recommended by the QUOROM standards for meta-analysis (Moher et al. 1999), we report results based on all available studies and results based only on published studies separately. In the text, studies described are published studies unless otherwise noted. In the tables, unpublished studies are collated separately. References for unpublished studies are included in the Appendix.
So that we report cumulative experience, we included studies from the previous review. These are indicated in italics in the tables (some of the names of the first authors and references for these previous studies have changed since the previous review). In addition, in this review we have located some studies missed by the first author in the previous review. We did not locate any foreign language articles that met our inclusion criteria.
Data extraction
The information from the articles was abstracted by the first author and verified by the second author. Discrepancies were reconciled with mutual agreement or, if necessary, further clarification from the original author. A draft of the paper was sent to the 40 authors whose studies were included in the main analyses (see Tables) to verify our citation or recalculation of their outcomes. Twelve responded.
Data synthesis
We initially intended to use meta-analytical methods to answer our major questions. However, in collating data for the meta-analysis we found that studies varied so widely in their methods that we believed a typical fixed-effects meta-analysis was not indicated (Rosenthal 1995; Egger et al. 2001). We could have conducted a random-effects meta-analysis (Rosenthal 1995) but chose not to do so due to (1) the methodological heterogeneity of studies, (2) the necessary extensive recalculation of data (see below) and (3) the purpose of our review was not to derive a point estimate of effect sizes per se but rather to determine whether or not effects occurred. As an alternative, we use a subjective rating of the consistency of the evidence across trials and the overall magnitude of any consistent effects to form conclusions. To illustrate the magnitude of any effects, we present the interquartile range (i.e. the 25th-75th percentiles). We do not report whether trials reported their results as statistically significant for two reasons. First, several studies did not report statistical significance even when reductions were very large (e.g. 50% or more). Secondly, many of the tests for statistical significance were not based on intent-to-treat samples, which is the basis for the calculations in the present analysis.
For the large majority of the outcomes we had to calculate derived outcomes (e.g. subtract final CPD from original CPD and represent as percentage). Data calculated by us are represented in bold type in the text and tables. Many studies excluded those with missing data from analysis, which is equivalent to assuming all missing data are equivalent to obtained data. Because many of these studies reviewed were more intensive clinical studies, we and others (Hall et al. 2001) believe it likely that those who are missing data in clinical studies are less likely to have been successful than those who provide data. Thus, when necessary, we recalculated outcomes using an intent-to-treat principle and assumed missing persons had returned to baseline values. Sometimes it was difficult to determine the intent-to-treat sample sizes from the text. As many of the studies had high dropout rates, some of our recalculated values differ dramatically from those cited in the article. We constructed tables only when there was a modicum of studies of similar methodology; however, this does not mean that studies cited only in the text were less important or less informative than those cited in the tables. For brevity, we have not cited individual studies in the text when the studies being referred can be discerned by examining the table. We distinguish among articles, studies and comparisons. A single study can produce multiple comparisons (e.g. if it has multiple experimental groups or multiple outcomes) and even multiple articles.
Outcome measures
Our major measures of reduction were percentage reduction in CPD from baseline smoking, absolute number of cigarettes foregone, incidence of achieving a ≥50% reduction in CPD and conversion to non-daily smoking. Which of these measures is optimal is debatable (Farkas 1999). One concern with percentage reduction outcomes is that to achieve a 50% reduction, a smoker who begins with 30 CPD has to give up more CPD than a smoker who begins with 10 CPD. Although this might suggest that heavier smokers would have more difficulty achieving a 50% reduction, epidemiological studies (reviewed below) found that heavier smokers were slightly more likely to reduce, reduced more CPD and were more likely to reduce ≥50% (Farkas 1999; Hughes, Cummings & Hyland 1999; Godtfredsen et al. 2001; Falba et al. 2004; Hyland et al. in press; Joseph et al. in press).
RESULTS
Aim 1: To what extent do smokers spontaneously reduce their smoking?
Introduction
Studies on spontaneous reduction can be divided into cross-sectional surveys, prospective studies and studies of non-daily smoking. The first refers to epidemiological surveys that could show a decline in CPD between serial independent surveys across points in time. The second refers to cohort studies of a defined, population-based sample that could show a decline in CPD in individual smokers over time. The third refers to studies that reported the prevalence or incidence of switching from daily to non-daily smoking. The previous review cited two studies on spontaneous quitting and we have located another 17 studies. All 19 of these studies have been published.
Cross-sectional studies
US cross-sectional epidemiological data suggest that CPD among continuing smokers increases with age until smokers are in their 40 s and then declines (Burns, Major& Shanks 2003a). Most large, serial cross-sectional surveys (e.g. US National Health Interview Survey, US California Tobacco Survey and the US Massachusetts state survey) indicate that smokers are smoking fewer CPD than in the past (National Cancer Institute 2000; Gilpin& Pierce 2002). For example, CPD among continuing smokers decreased by 20% from 1980 to 1998 in the National Health Interview Surveys (Burns et al. 2003a). On the other hand, the US Current Population Survey did not find a reduction in CPD between 1992 and 1996 (Burns et al. 2003b). Among UK adult smokers (West et al. 2001), 29% of smokers stated that they had cut down (amount unspecified) in the previous year, but not as an attempt to quit.
Prospective studies
We located nine articles reporting on six prospective studies of population-based samples that present their results in a manner such that they can be aggregated (Table 1). Six articles were based on population-based, no-intervention, prospective studies (Table 1). One article examined older US smokers (Falba et al. 2004); one examined smokers in a US state that had a vigorous tobacco control program (Farkas 1999); three describe cohort samples of Danish smokers (Godtfredsen et al. 2002a, b, 2003) and one was on German smokers (Meyer et al. 2003).
Table 1.
Studies of spontaneous smoking reduction.a
| Study | Sample | Longest follow-up (years) | % (CPD) reducedb | % Achieved ≥50% reductionb |
|---|---|---|---|---|
| Non-intervention studies | ||||
| Falba et al. 2004 | 2064 51–61-year-old US smokers | 2 | 5% (1) | 5% |
| Farkas 1999 | 1682 ≥ 18-year-old CA smokers | 1 | 9% (2) | 11% |
| Godtfredsen et al. 2002a, b, 2003 | 19 423 ≥ 20-year-old Danish smokersc | 16d | 10%e | |
| Meyer et al. 2003b | 836 ≥ 18-year-old German smokers | 0.6 | 1% (1) | |
| Minimal intervention studies | ||||
| Hughes et al. 1999 | 1584≥18-year-old US/Canadian smokers in COMMIT | 2 | 5%(1) | 7% |
| Hyland et al. in press | 3385 ≥ 18-year-old US/Canadian smokers in COMMIT | 5 | 10% | |
| Pisinger et al. 2005 | 1276 ≥ 30-year-old Danish smokers | 1 | 0% (0) | 2% |
| 25th-75th percentile | 1276–3385 | 1–5 | 1–5% (1–1) | 5–10 |
CA = California, USA; COMMIT = Community Intervention Trial; CPD = cigarettes per day, bold type = from our calculations; italics = studies in previous review; see text for other studies.
Does not include abstainers.
Varied from 19 423 to 19 732 across analyses.
Varied from 13.8 to 15.5 years across analyses.
Varied from 10 to 11% across analyses.
Three articles do not report on non-intervention studies, but on population-based samples in a randomized controlled trial (RCT). Two of these articles reported on US/Canadian smokers in a study that intervened not on individuals but on policy change, and had a very small effect on smoking (Hughes et al. 1999; Hyland et al. in press). The third article reported outcomes in a population-based no-treatment group in a minimal intervention smoking cessation trial (Pisinger et al. 2005). All the above articles had large population-based samples with long-term follow-ups. We did not include smokers who became abstinent as reduced smokers in this analysis, because our question concerns only continuing smokers.
Among these studies, the mean decline in CPD among continuing smokers was small and clinically insignificant (Table 1). In addition, few smokers decreased their smoking by ≥50%.
Studies of non-daily smoking
The results from the four studies of smokers switching from daily to non-daily smoking could not be aggregated into a table as they had very different timeframes, methods, reporting styles, etc. Among US smokers, about 10% of previous daily smokers had converted to non-daily smokers by the time of the interview (Hassmiller et al. 2003). Among Swedish adult daily smokers, 6.5% had become ‘intermittent smokers’ (undefined) a year later (Lindstrom & Isacsson 2002). Among Californian daily smokers, over a 20-month period, 3.2% of smokers became non-daily smokers and 2.3% became daily smokers of ≤5 CPD (Zhu et al. 2003). Among adult smokers in Minnesota work-sites, 9% of daily smokers had became non-daily smokers over the previous 2 years (Hennrikus, Jeffery & Lando 1996). Based on these surveys, we estimate that about 1–7% of daily smokers convert to non-daily smoking over a 12-month span. Such conversions may be increasing, as the US Behavioral Risk Factor Survey (Porter et al. 2003) and other US surveys are showing a dramatic rise in non-daily smoking over time.
Discussion
In adult daily smokers who continue to smoke daily, CPD appears to decrease very slightly over time. In addition, only a small minority of smokers report large reductions in CPD (i.e. ≥50% reduction) or switch to non-daily smoking.
The existing data on spontaneous reduction leave several gaps. Importantly, none of the studies documented exactly how long reductions at follow-up had been maintained. None reported whether reductions were a reaction to tobacco control programs, events such as moving to a job with smoking restrictions, increased taxes, becoming ill, etc. Also, the surveys did not report whether reductions were (a) the ultimate goal, (b) in preparation for quitting or (c) sequelae of a failed quit attempt. Finally, although only a small minority of daily smokers reduce by ≥50% or switch to non-daily smoking (i.e. 1–10%), it should be remembered that only 2–4% of daily smokers quit each year (National Cancer Institute 2000); thus, it may be that more daily smokers reduce by ≥50% or switch to non-daily smoking each year than quit.
Aim 2: To what extent do smokers who try to quit and fail return to smoking less?
Introduction
Over 80% of quit attempts fail (Hughes, Keely & Naud 2004a), even with the best of treatment (Fiore et al. 2000). Those who fail may return to a lower number of CPD because they believe reduced smoking is less harmful, or that it will be easier to quit on their next quit attempt or because they have lost some of their nicotine tolerance (Perkins 2002) and thus require a lower dose of nicotine. Although we present several studies on reduction in cessation failures, the Discussion section outlines why we believe this set of studies is perhaps biased.
Studies of treatment failures
To be included in this analysis, a study had to recruit smokers who were attempting to stop smoking and had to mention in the title or abstract that the CPD in continuing smokers was reported. Overall, the previous review reported six studies and we located another seven studies. Nine of the 13 studies are published.
Among the 10 trials located whose data could be aggregated (Table 2), four of the trials tested a behavioral therapy (Lichtenstein & Rodrigues 1977; Lando & McGovern 1982; Glasgow et al. 1989; Becona & Garcia 1993) and six a medication (Fornai et al. 1996; Hughes et al. 1981; Norregaard et al. 1992; Hurt et al. 2003; Jolicoeur et al. 2003; Hughes et al. 2004b). All but one (Jolicoeur et al. 2003) were RCTs.
Table 2.
Studies of cessation treatment failures.a
| Study | Sample size | Longest follow-up (months) | % (CPD) reduced |
|---|---|---|---|
| Behavior therapy | |||
| Becona & Garcia 1993 | 47 | 12 | 37% (7) |
| Glasgow et al. 1989 | 53 | 6 | 22% (5) |
| Lando & McGovern 1982 | 100 | 36 | 18% (6) |
| Lichtenstein & Rodrigues 1977b | 117 | 24–72b | 41%b |
| Nicotine replacement therapy | |||
| Fornai et al. 1996c | 242 | 40 | 14% (3) |
| Hughes et al. 1981 | 2449 | 48 | 16% (6) |
| Hughes et al. 2004bd | 1722 | 12 | 28% (9) |
| Hurt et al. 2003 | 397 | 8 | 9% (3) |
| Jolicoeur et al. 2003 | 173 | 6 | 17% (8) |
| Norregaard et al. 1992 | 259 | 12 | 4% (2) |
| 25th-75th percentile | 100–397 | 12–40 | 14–28% (3–7) |
CPD = cigarettes per day; bold type = from our calculations; italics = studies in previous review; see text for other studies.
Combines outcome across four trials; CPD not calculable.
Unpublished study.
Updated analysis of study cited in previous review.
All but one of the studies in Table 2 reported post-failure reduction aggregated across active and control groups. The one exception found no difference in reduction between bupropion and placebo groups when retreated after cessation failure (Hurt et al. 2003). On the other hand, two unpublished studies, which did not report data in a manner that could be included in Table 2, both found more reduction in the bupropion than placebo group among treatment failures (Gonzales, Durcan& Johnston 2004; Wileyto, Heitjan & Lerman 2004).
The percentage reduction was small in two studies (range = 4–9%) and substantial in the other eight studies (14–41%; Table 2). The one unpublished study (Fornai et al. 1996) appeared to find results similar to the published studies. The only study to report the incidence of large reduction found 30% reduced CPD by ≥50% (Hughes et al. 2004b). Importantly, in all four studies that reported on maintenance of reduction, the amount of reduction became smaller (i.e. participants trended back to base rates) as time post-relapse increased (Hughes et al. 1981; Becona & Garcia 1993; Hurt et al. 2003; Hughes et al. 2004b). One other study reported that treatment failures who returned to a lower rate of CPD did not maintain this reduction; however, this study did not report baseline smoking. Thus, it is unclear if lower rate smokers post-failure had always smoked less or had reduced (Hill et al. 1988).
Discussion
These results indicate that smokers who fail to quit return to lower CPD but that this reduction dissipates over time. However, this conclusion should be considered tentative, because our list of studies is incomplete and because our results may overestimate the amount of reduction. The list is incomplete because our anecdotal observation is that some cessation studies report on CPD among failed quitters in the text but do not mention this in their title or abstract, and thus we would not detect such studies. In addition, as reporting CPD in treatment failures is treated as optional, reduction in treatment failures is probably more likely to be reported than non-reduction.
Aim 3: To what extent can smokers substantially reduce and maintain reductions via pharmacological and behavioral treatments?
Introduction
At any given time, >80% of smokers are not seriously planning on quitting in the next 30 days (Wewers et al. 2003). Although motivational interventions for such smokers appear useful (Spencer et al. 2002; Riemsma et al. 2003), many researchers have examined reduction as a goal for such smokers either in the hope of reducing their risks from smoking or of prompting later cessation. The previous review located seven such studies and we located another 19 studies. Twenty-one of the 26 studies are published.
Nicotine replacement therapy (NRT) trials
We located 19 trials that tested NRT for reduction in smokers not currently trying to quit whose data could be aggregated (Table 3) (Rennard et al. 1990, 1994; Fager-strom et al. 1997; Bolliger et al. 2000; Hurt et al. 2000; Fagerstrom, Hughes & Callas 2002; Jimenez-Ruiz et al. 2002; Kralikova, Kozak & Rasmussen 2002; Rennard et al. 2002; Stein et al. 2002; Carpenter, Hughes & Keely 2003; Haustein et al. 2003; Landfeldt et al. 2003; Wennike et al. 2003; Carpenter et al. 2004; Etter, Laszlo & Perneger 2004; Hecht et al. 2004; Joseph et al. 2004; Kotlyar et al. 2004). None of the trials stated that those in the reduction group were instructed not to quit and many trials stated explicitly that those in the reduction group were encouraged to quit at any point during reduction.
Table 3.
Methods of studies of nicotine replacement to help smokers not trying to quit to reduce.a
| Study | Study design | Intent-to-tx sample size | Duration of tx (months) | Control condition | Longest lollow-up (months) |
|---|---|---|---|---|---|
| Published studies | |||||
| Nicotine gum | |||||
| Jimenez-Ruiz et al. 2002b | PP | 17 | 6 | Within-Ss | 18 |
| Rennard et al. 1990 | PP | 15 | 2 | Within-Ss | 2 |
| Wennike et al. 2003 | RCT | 411 | 12 | Placebo | 24 |
| Nicotine inhaler | |||||
| Bolliger et al. 2000 | RCT | 400 | 18 | Placebo | 24 |
| Fagerstrom et al. 2002c | PP | 39 | 2 | Within-Ss | 2 |
| Hurt et al. 2000 | PP | 23 | 6 | Within-Ss | 6 |
| Stein et al. 2002 | RCT | 51 | ? | No tx | 3 |
| Choice of NRTs | |||||
| Carpenter et al. 2003 | RCT | 67 | 1 | Cessation advice | 6 |
| Carpenter et al .2004 | RCT | 616 | 1.5 | No tx | 5 |
| Cessation advice | 6 | ||||
| Etter et al. 2004 | RCT | 923 | 6 | Placebo | |
| No tx | 26 | ||||
| Fagerstrom et al. 1997d | PP | 170 | 1 | Within-Ss | 1 |
| Hecht et al. 2004e | PP | 49 | 6 | Within-Ss | 6 |
| 25th-75th percentile for published studies | 39–411 | 1.5–6.0 | 3–18 | ||
| Unpublished studies | |||||
| Nicotine gum | |||||
| Haustein et al 2003f | RCT | 192 | 9 | Placebo | 12 |
| Landfelt et al 2003 | RCT | 364 | 12 | Placebo | 13 |
| Rennard et al. 1994 | RCT | 90 | ? | Placebo | 6 |
| Nicotine inhaler | |||||
| Rennard et al. 2002 | RCT | 429 | 12 | Placebo | 15 |
| Choice of NRTs | |||||
| Joseph et al. 2005 | RCT | 152 | 18 | Usual care | 6 |
| Kotlyar et al. 2004 | RCT | 151 | 3 | Within-Ss | 6 |
| Kralikova et al. 2002g | RCT | 314 | 6 | Placebo | 12 |
| 25th-75th percentile for all studies | 50–406 | 2–12 | 6–14 |
NRTs = nicotine replacement therapies, PP = pre- versus post-treatment comparison, RCT = randomized controlled trial, Ss = subjects, Tx = treatment; bold type = from our calculations; italics = studies in previous review; see text for other studies.
Examined self-selected groups.
Randomized to choice of versus assignment to different NRTs.
Only used waiting list group which later received NRT.
Intent of participants unclear.
Only used long-term induction group.
Smokers given choice of quitting or reducing.
One trial compared NRT for reduction versus cessation in smokers unwilling to quit but was not included because it reported only aggregate data for both outcomes (Pisinger et al. 2005). Several studies have examined the ability of less-risky cigarettes to reduce smoking in smokers not trying to quit. Although these products could be considered a NRT, we did not include studies of these products because their outcomes have been reviewed in previous articles and books (Institute of Medicine 2001).
Six trials examined nicotine gum alone, five examined inhaler alone and eight allowed participants to choose which NRT to use. None examined nicotine lozenge, microtab or patch. Most studies (11) allowed participants to use the medication for 6 months or longer. The presence or content of any adjunctive psychosocial treatment was not described in most (15) studies. Thirteen of the studies were parallel-group RCTs. Comparison groups received placebo in eight studies, no treatment in three studies and cessation advice or usual care in three studies. Six trials were not parallel-group RCTs but rather within-subjects comparisons of treatment versus baseline smoking.
Most trials were large (12 had n = 90) and most (15) had follow-ups of 6 months or longer. In six trials, the last follow-up was at the end of medication treatment; in seven it was 1–6 months after treatment ended, in four it was 12–20 months after treatment ended and in two it was unclear. In our analyses of these studies, we included abstainers (i.e. counted them as 100% reducers) because most trials only reported results in this manner and we could not recalculate outcomes excluding abstainers.
Fourteen NRT trials reported the mean reduction in CPD in each group. The percentage reduction with the intervention at longest follow-up was small (6% or two CPD) in one condition, moderate (18–45% or 8–10 CPD) in nine conditions and large (53–75% or 14–32 CPD) in four conditions (Table 4).
Table 4.
Results of studies of nicotine replacement to help smokers not trying to quit to reduce.a
|
% (CPD) reducedb |
% Achieved ≥ 50% reductionb |
|||||
|---|---|---|---|---|---|---|
| Study | Active | Control | RR | Active | Control | ORc |
| Published studies | ||||||
| Nicotine gum | ||||||
| Jimenez-Ruiz et al. 2002 | 23% (10) | 29% | ||||
| Rennard et al. 1990 | 63%(32) | |||||
| Wennike et al. 2003 | 18% (4) | 13% (3) | 1.5 | 6% | 1% | 13.9 |
| Nicotine inhaler | ||||||
| Bolliger et al. 2000 | 10% | 3% | 3.4 | |||
| Fagerstrom et al. 2002 | 66% (14) | +9% (+2) | Larged | |||
| Hurt et al. 2000 | 25% (10) | 13% | ||||
| Stein et al. 2002 | 75% (27) | 4% (2) | 18.8 | |||
| Choice of NRTs | ||||||
| Carpenter et al. 2003 | 34% (8) | |||||
| Carpenter et al. 2004 | 43% (10) | 17% (4) | 2.5 | 39% | 15% | 3.6 |
| Etter et al. 2004 | 33% (10) | 26% (8) | 1.3 | 31% | 22% | 1.6 |
| 25%(8) | 1.3 | 24% | 1.4 | |||
| Fagerstrom et al. 1997 | 45% (10) | 50% | ||||
| Hecht et al. 2004e | 32% (8) | |||||
| 25th-75th percentile for published studies | 25–63% | 4–25% (2–8) | 1.3–18.8 | 12–35% | 3–22% | 1.6–3.6 |
| Unpublished studies | ||||||
| Nicotine gum | ||||||
| Haustein et al. 2003 | 6% | 0% | 13.0 | |||
| Landfelt et al. 2003 | 6% (2) | 8% | 3% | 3.1 | ||
| Rennard et al. 1994a | 53%(23) | 49%(21) | 1.1 | |||
| 56%(25) | 49%(21) | 1.1 | ||||
| Nicotine inhaler | ||||||
| Rennard et al. 2002 | 8% | 2% | 4.6 | |||
| Multiple NRTs | ||||||
| Joseph et al. 2005 | 39% (11) | 25% (7) | 1.6 | |||
| Kotlyar et al. 2004 | 27%e | |||||
| Kralikova et al. 2002 | 36% | 27% | 1.5 | |||
| 25th-75th percentile for all studies | 25–56% (8–23) | 13–26% (3–8) | 1.3–2.5 | 8–27% | 2–22% | 1.6–4.6 |
BUP = bupropion, CA = cessation advice, CPD = cigarettes/day, NRT = nicotine replacement therapy, NT = no treatment, PL = placebo, PREP = potential reduced exposure product, OR = odds ratio, RR = reduction ratio (see text); bold type = from our calculations; italics = studies in previous review; see text for other studies.
Includes abstainers.
OR underlined controlled for other factors.
Control group increased CPD; thus, RR not calculable.
40% reduction.
Seven of the studies that reported mean reduction were RCTs of NRTs. For these studies, we calculated a relative risk ratio of the percentage reduction in the active group divided by the percentage reduction in the no-treatment or placebo control group. Across the nine active NRT versus placebo comparisons this ratio was always greater than 1.0, i.e. active NRT always produced more reduction than placebo. The magnitude of the ratio was negligible (1.1) in two comparisons, small (1.3–1.6) in four comparisons and large (>2.5) in three comparisons. For the other five studies that were not RCTs but within-subjects studies, the CPD with NRT treatment was always less than baseline smoking.
Eleven NRT studies reported the incidence of achieving a reduction in CPD of ≥50% in each group. In the 13 active treatment conditions, the incidence of large reductions was small (range = 6–13%) in six conditions and was moderate (27–50%) in the other seven conditions. Eight of these were RCTs. Either the authors or ourselves calculated an odds ratio (OR) of achieving ≥50% reduction for the active versus control conditions. The OR favoring NRT was always greater than 1.0 and was moderate (1.4–1.6) in three comparisons and large (3.1–13.9) in five comparisons. One other NRT study presented results in a format different from Table 4. This study reported that 70% of the 27 smokers given either nicotine or placebo gum reduced by ≥30% over a 2-month period (Rennard et al. 1992). The amount of reduction and the increase with NRT did not appear to differ between (a) published versus unpublished studies, (b) participants who could have been using NRT at the follow-up versus not, (c) types of NRT or (d) smokers who were assigned to a particular type of NRT versus were allowed to choose the type of NRT. However, within one study, participants had greater reduction when allowed to choose their NRT than when randomly assigned a NRT (Fagerstrom et al. 1997).
In terms of maintenance of effects, most of the studies that reported the incidence of large reduction used a criterion that the reductions must be observed at all interim follow-ups over long periods of time (6–24 months) (Bolliger et al. 2000; Kralikova et al. 2002; Rennard et al. 2002; Haustein et al. 2003; Landfeldt et al. 2003; Wennike et al. 2003). To illustrate the degree to which reducers can maintain reductions, we plotted the percentage of smokers who maintained a ≥50% reduction in CPD at 3–4-, 6- and 12-month follow-ups in the two studies that reported such data (Bolliger et al. 2000; Wennike et al. 2003) (Fig. 1). For simplicity we plotted only those in the NRT group. In both studies, these smokers had access to NRT for the entire period. For comparison, we used data from a recent meta-analysis (Fagerstrom 2003) and plotted the percentage of smokers trying to quit who maintained abstinence at the same follow-ups. Again, we used only those in the NRT group. The prevalence of maintenance of abstinence was similar to that for reduction in one study and slightly greater than that for the other study. Although such cross-study comparisons can be misleading, it appears the ability to maintain large reductions in CPD is roughly similar to the ability to maintain abstinence.
Figure 1.
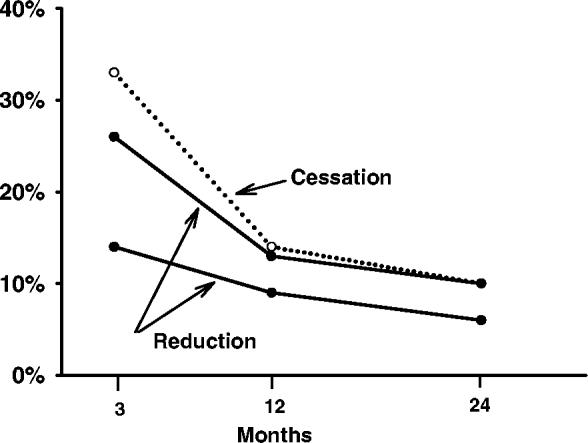
Percentage of smokers in two studies of smokers not trying to reduce on nicotine replacement who maintained ≥50% reduction in cigarettes/day over time (solid lines, solid circles) versus percentage of smokers trying to quit on nicotine replacement who maintained abstinence over time taken from a recent meta-analysis (dotted lines, open circles) (see text for references)
Two studies compared a reduction intervention to an active cessation-advice treatment group (cessation advice) in smokers who were not currently interested in quitting (Carpenter et al. 2003, 2004). In both studies the reduction group reduced slightly, but not significantly, more than the cessation advice group (Carpenter et al. 2003, 2004). A third study, not included in the tables, also compared reduction with cessation advice in asthmatic smokers (Tonnesen et al. 2005). However, in this study some of the smokers wished to reduce and some wished to quit. Although not directly comparable to the other studies, we included this study for completeness. In this mixed population, 30% in the reduction group reduced (from 20 to 7 cigarettes/day) at 4-month follow-up versus 38% in the cessation advice group (significance not stated).
Bupropion
Only one study has examined bupropion for reduction in smokers not trying to quit and used a somewhat different design from the NRT studies (Hatsukami et al. 2004). In this large (n = 594) RCT of bupropion versus placebo, smokers who decided to quit during the study entered a second protocol and this second protocol had different follow-up times from the main protocol. Among those who did not try to quit, the mean percentage reduction over the first 3 months was about 20% in both the bupro-pion and placebo groups. The incidence of large (≥50%) reduction was about 18% in both groups at 3-month follow-up. At 1-year follow-up, only the incidence of large reduction among continuing smokers was reported and this was 8% in the bupropion group and 12% in the placebo group (P = NS).
Psychosocial interventions
The previous review examined three studies that tested formal behavioral interventions for reduced smoking in smokers not trying to quit. The current review adds two more. Among these five studies, four are published. Two of the trials were conducted in the 1980s and the other three were after 2000 (Table 5). All had sample sizes of ≤60/group. None of the five were RCTs; all used within-subject designs. All five tested behavioral therapies. Most focused on either eliminating certain cigarettes or increasing the interval between cigarettes. Two used computer-aided reduction and two used very brief interventions. One trial also used a medication in both of the psychosocial groups being compared, but the medication use in this trial was minimal (Riggs, Hughes & Pillitteri 2001). Three trials had follow-ups of ≥6 months.
Table 5.
Methods of psychosocial interventions to help smokers not trying to quit to reduce.a
| Study | Intent-to-tx sample size | Study design | Tx | Total tx time (min)/duration (wks) | Longest follow-up (months) |
|---|---|---|---|---|---|
| Published studies | |||||
| Early studies | |||||
| Glasgow et al. 1983 | 9 | MBL | MM/HR, SR | 350/8 | 6 |
| Glasgow et al. 1985 | 60 | PPb | MM/HR, SR | 250/5 | 30 |
| Recent studies | |||||
| Riggs et al. 2001 | 20 | PP | HR | 40/2 | 1 |
| 20 | SR | 40/2 | |||
| Riley et al. 2002 | 44 | RT | CASR | 20/7 | 12 |
| 49 | CAHR | 20/7 | |||
| Unpublished recent study | |||||
| Brue 2002 | 47 | PP | CASR | ?/4 | 1 |
| 25th-75th percentile for all studies | 20–49 | 1–12 |
CA=computer-aided, HR= hierarchical reduction of eliminating easiest cigarettes to reduce, MBL= multiple baseline within-subjects comparison, Min = minutes, MM = multiple methods (change nicotine yield, topography, cigarette length), Mo = months, PP = pre-versus post-comparison, RT = randomized trial of two active treatments, SR = scheduled reduction based on increasing interval between cigarettes, Tx = treatment, Wks = weeks, WSCT = within-subjects crossover trial; bold type = from our calculations; italics = studies in previous review.
Actually a randomized trial but did not report long-term outcome for control group.
All five studies reported mean reduction in CPD. The difference between baseline and treatment conditions was small to moderate (range = 13–35%, four to 12 CPD) in the two early studies and was moderate (range = 21–45%, six to 12 CPD) in the five conditions of the three more recent studies (Table 6). The two studies with long-term follow-up found that reductions were well maintained over 12–30 months (Glasgow et al. 1985; Riley et al. 2002). Five conditions reported the incidence of large (≥50%) reductions. One condition found a small incidence (16%), two found moderate incidences (30%) and two found large incidences (50–58%).
Table 6.
Results of psychosocial interventions to help smokers reduce.a
| Study | % (CPD) reducedb | % Achieved ≥ 50% reductionb |
|---|---|---|
| Published studies | ||
| Early studies | ||
| Glasgow et al. 1983 | 13% (4) | |
| 35% (12) | ||
| Recent studies | ||
| Riggs et al. 2001 | 38% (10) | 30% |
| 45% (12) | 50% | |
| Riley et al. 2002 | 23% (6) | 30% |
| 21% (6) | 16% | |
| Unpublished recent study | ||
| Brue 2002 | 41% (10) | 58% |
| 25th-75th percentile for all studies | 21–41% (6–12) | 30–50% |
CPD = cigarettes per day; bold type = from our calculations; italics = studies in previous review.
Includes abstainers.
Because none of the studies included a control group per se, RR or OR for active versus control conditions could not be calculated; however, all five studies found fewer CPD with intervention than at baseline. The single unpublished study appeared to find results similar to the published studies.
Discussion
Across the NRT studies, NRT always produced more reduction than placebo or no-treatment control groups. This occurred across type of NRT, unpublished versus published studies, study design, duration of follow-up and type of control condition. Thus, we conclude NRT consistently helps smokers not trying to quit to make reductions in CPD. This conclusion is similar to those of previous reviews (Zellweger 2001; Jimenez-Ruiz et al. 2002; Warner 2003; Tonnesen, in press).
One limitation of the current NRT data set is that several of the studies (seven of 19) were available only in an unpublished format, which limited our ability to critique their quality. However, results based only on published studies were similar to those based on all studies. Another limitation is that few of the medication trials described the extent of instructions or adjunct psychosocial treatment; thus, the feasibility of these approaches for medical practices is unclear. Also, none of the trials have tested nicotine lozenge, microtab or patch per se as a treatment.
Our estimate of the magnitude of reduction with NRT is probably a significant underestimate because many studies had a large loss-to-follow-up and we assumed that all dropouts had returned to baseline smoking rates. Even so, the magnitude of reduction found in most of these studies and the fact that most reduction was maintained for periods of 6 months or longer suggests that reduction with NRT is a robust phenomenon.
The single study of a non-nicotine medication—bupropion—reported no greater reduction with bupro-pion than placebo. In addition, even though bupropion is given for 1–2 weeks prior to cessation, we could not locate empirical data on whether such pretreatment reduces CPD prior to the quit date.
In the behavioral treatment studies, although the magnitude of effects was substantial, the studies were few in number and had small sample sizes (n ≤ 60). None included a control group followed over time. As a result, although the data are promising, the efficacy of psycho-social treatments cannot be concluded definitively. Thus, true RCTs of psychosocial treatments are needed. Whether behavioral treatments would be more effective if a background of medication were present or vice versa has not been tested; thus, factorial studies of adding psychosocial therapies to medications or vice versa are also indicated.
Aim 4: To what extent do smokers compensate when they reduce?
Introduction
Compensation (i.e. regulation or titration) refers to increases in smoking behavior when smokers face reduced nicotine intake (Scherer 1999) and is due to an attempt to maintain nicotine levels. For example, when smokers reduce their CPD, they take more and bigger puffs, block vent holes, etc. (National Cancer Institute 2001). One way to test for compensation is to determine whether reductions in measures of smoke exposure are less than reductions in CPD.
Our compensation analysis focuses only on the medication and behavioral intervention studies, because none of the population-based studies reported on compensation and the studies of relapsers reported compensation results that varied widely. For example, among the relapser studies, the early Multiple Risk Factor Intervention Trial (MRFIT) analysis of CPD showed almost complete compensation (Hughes et al. 1981), whereas two later studies of relapsers suggested no compensation whatsoever (Glasgow et al. 1989; Hughes et al. 2004b). In contrast, the studies of pharmacotherapy and behavioral interventions for reduction in smokers not trying to quit reported more homogeneous outcomes and will be the focus of this section. The previous review reported six studies examining compensation and this review adds another nine studies. Twelve of the 15 studies are published.
The available markers of smoke intake that can be used for compensation analyses are carbon monoxide (CO), cotinine and thiocyanate, each of which has problems (SRNT Subcommittee on Biochemical Verification 2002). The major problem with CO is that it has a short half-life and thus samples only recent smoking, and can be influenced by a recent bout of smoking. Cotinine has a longer half-life but is influenced by NRT. Thiocyanate also has a longer half-life but is less sensitive and specific than cotinine or CO. Markers of cancer and cardiovascular disease are not feasible because their relationship to smoke intake is either weak or has not been determined fully (Hatsukami et al. 2003). Among the 15 studies, 14 reported CO levels and one reported cotinine.
Medication intervention studies
We found nine studies of NRT and one of bupropion exposure marker data (Table 7). To examine compensation, we first report the percentage reduction in marker. Then we calculate ‘percentage compensation’ by comparing the percentage reduction in the marker versus the percentage reduction in CPD using the formula:
With this index, if one fully compensated (i.e. no reduction in the marker) the percentage compensation would be 100%, and if one did not compensate at all (i.e. percentage reduction in CPD = percentage reduction in the marker), it would be 0%. For this analysis we excluded abstainers because as they are not smoking, they cannot be compensating.
Table 7.
Exposure marker results of interventions to help smokers reduce.a
|
% (CO in ppm) reduction in marker |
Compensation |
|||
|---|---|---|---|---|
| Study | Active | Control | Active | Control |
| Published studies | ||||
| Nicotine gum | ||||
| Jimenez-Ruiz et al. 2002 | 14% (4) | 39% | ||
| Rennard et al. 1990 | 44% (21) | 30% | ||
| Wennike et al. 2003 | 15% (4) | 8% (2) | 17% | 39% |
| Inhaler | ||||
| Fagerstrom et al. 2002 | 46% (13) | 30% | ||
| Hurt et al. 2000 | 10% (3) | 59% | ||
| Choice of NRTs | ||||
| Carpenter et al. 2003 | 29% (8) | 15% | ||
| Fagerstrom et al. 1997 | 29% (7) | 36% | ||
| Bupropion | ||||
| Hatsukami et al. 2004b | 18% | 2% | 36% | 80% |
| Psychosocial Tx | ||||
| Glasgow et al. 1983 | 26% (14) | 0% | ||
| Glasgow et al. 1985 | 19% (8) | 46% | ||
| Riggs et al. 2001 | 19% (8) | 50% | ||
| 19% (8) | 58% | |||
| Riley et al. 2002b | 10% (2) | 57% | ||
| 10% (2) | 52% | |||
| 25th – 75th percentile for published studies | 14–29% (4–8) | 2.8% (2–2) | 30–52% | 39–80% |
| Unpublished studies | ||||
| Nicotine gum | ||||
| Landfelt et al. 2003 | 4% (1) | 33% | ||
| Rennard et al. 1994a | 28% (12) | 25% (9) | 47% | 49% |
| 20% (4) | 25% (9) | 64% | 49% | |
| Brue 2002b | 20% (6) | 50% | ||
| 25th-75th percentile for all studies | 14–28% (4–8) | 5–25% (2–9) | 30–52% | 44–65% |
CO = carbon monoxide, NRTs = nicotine replacement therapies; ppm = parts per million. Tx = treatment, bold type = from our calculations; italics = studies in review; see text for other studies.
Used cotinine instead of carbon monoxide as marker.
Among the 10 NRT conditions that examined smoke exposure, the reduction in the marker was small (range = 4–15%) in three conditions and was moderate (20–46%) in seven conditions (Table 7). The percentage compensation in the 10 NRT conditions was large (47–64%) in three conditions and moderate (17–39%) in the remaining seven conditions. Overall, the reduction in CO was about a third less than the reduction in CPD (Table 7). One might expect compensation to be less when smokers receive NRT and data from one published study suggest this finding; i.e. 7% for NRT versus 39% for placebo (Wennike et al. 2003), but data from another unpublished study indicate similar compensation with NRT and placebo (Rennard et al. 1994).
The single study of bupropion (Hatsukami et al. 2004) reported cotinine rather than CO levels. Although the reduction in CPD was similar in bupropion and placebo groups, the reduction in cotinine was 14% in the bupro-pion group versus 4% in the placebo group. As a result, compensation appeared to be less in the bupropion group (36%) than in the placebo group (80%).
Behavioral intervention studies
Among the five behavioral intervention studies, three measured CO and two measured cotinine. In terms of compensation, in the first study (Glasgow et al. 1983) the reduction in CO was actually greater than the reduction in CPD, resulting in 0% compensation. This may be because this study also taught smokers to smoke less intensely, to change to lower CO yield cigarettes, etc. or the small (n = 9) sample size (Table 7). In the other six psychosocial conditions, compensation was homogeneous with reductions in CO or cotinine of about half that expected from reductions in CPD.
To determine whether those who reduced more had more compensation, we computed correlation coefficients between amount of reduction and amount of compensation across NRT and behavioral treatment studies. We omitted one study as an outlier because it found greater reductions in CO than CPD (Glasgow et al. 1983). The percentage reduction in CPD was not correlated significantly with percentage compensation; thus, greater reductions in CPD did not result in more compensation.
Discussion
These results based on medication and behavioral treatment studies indicate that compensation does occur; thus, relying on CPD to estimate exact reduction in smoke exposure is problematic. On the other hand, compensation is far from complete, such that substantial reductions in tobacco smoke exposure still occur. For example, compensation was almost always <50% of the reduction in CPD. The compensation results were fairly consistent across trials. One of the anticipated advantages of using NRT is that NRT should abate compensation. Surprisingly, only two studies allowed a direct test of this and they produced inconsistent results. Thus, more direct tests of this notion are indicated.
Readers need to be aware of three possible biases in our compensation results. First, even in studies on research wards with tight experimental control, the correlation of CO (the most common marker) versus CPD is<0.50 (Hatsukami et al. 2003), indicating that CO is a less-than-perfect marker of smoke intake (SRNT Subcommittee on Biochemical Verification 2002). Secondly, as mentioned earlier, several of the studies had a large loss to follow-up and, thus, our intent-to-treat recalculations dramatically reduced their estimates of the amount of reduction in smoke exposure. This would suggest that our estimates of exposure reduction may be underestimates and our estimates of compensation overestimates. Thirdly, although we have presented a ratio of decrease in marker to decrease in CPD as a measure of compensation, if a participant exaggerated his/her reduction in CPD, then this would inflate the difference in reduction in CPD versus reduction in CO percentage, suggesting more compensation than actually occurred.
CONCLUSIONS
Overall, the results of new studies strengthened the conclusions of the previous review that smoking reduction is feasible when aided by treatment. Specifically, the review suggests that (1) little spontaneous reduction occurs, (2) among those who try to stop smoking and relapse, some return to fewer CPD but whether they maintain this reduction is unclear, (3) smokers in treatment programs make significant reductions in their smoking and maintain these over time and (4) some compensatory smoking occurs with reduction but significant declines in carbon monoxide exposure still occur. In addition, the new studies reviewed show more clearly that (5) nicotine replacement (and perhaps psychosocial treatments) are active treatments that aid in smoking reduction. Whether smoking reduction decreases the harm from smoking or increases or decreases future quitting will be covered in a separate paper.
Acknowledgements
Preparation of this report was funded by grant DA-11557 (J.H.) and Senior Scientist Award DA-00490 (J.H.) from the US National Institute on Drug Abuse. We thank Peter Callas, Laura Solomon, Dorothy Hatsukami and Robert West for their comments on earlier drafts.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edn American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Becona E, Garcia MP. Nicotine fading and smoke-holding methods for smoking cessation. Psychological Reports. 1993;73:779–786. doi: 10.2466/pr0.1993.73.3.779. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, III, Kozlowski LT, Yu L. Influence of smoking fewer cigarettes on exposure to tar, nicotine, and carbon monoxide. New England Journal of Medicine. 1986;315:1310–1313. doi: 10.1056/NEJM198611203152102. [DOI] [PubMed] [Google Scholar]
- Bolliger CT, Zellweger JP, Danielsson T, van Biljon X, Robidou A, Westin A, et al. Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety. BMJ. 2000;321:329–333. doi: 10.1136/bmj.321.7257.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P, Gandini S, Robertson C, Zatonski W, Fagerstrom K-O, Slama K, et al. Characteristics of smokers’ attitudes towards stopping. European Journal of Public Health. 2000;10:5–14. [Google Scholar]
- Burns DM, Major JM, Anderson CM, Vaughn JW. In: Those Who Continue to Smoke: Is Achieving Abstinence Harder and Do We Need to Change Our Interventions? Smoking and Tobacco Control Monograph no. 15. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda, MD: 2003b. Changes in cross-sectional measures of cessation, numbers of cigarettes smoked per day, and time to first cigarette—California and national data; pp. 101–125. [Google Scholar]
- Burns DM, Major JM, Shanks TG. In: Those Who Continue to Smoke: Is Achieving Abstinence Harder and Do We Need to Change Our Interventions? Smoking and Tobacco Control Monograph no. 15. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda, MD: 2003a. Changes in number of cigarettes smoked per day: cross-sectional and birth cohort analyses using NHIS; pp. 83–100. [Google Scholar]
- Carpenter MJ, Hughes JR, Keely J. Effect of smoking reduction on later cessation: a pilot experimental study. Nicotine and Tobacco Research. 2003;5:155–162. doi: 10.1080/146222003100007385. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction and motivational advice increase future cessation among smokers not currently planning to quit. Journal of Consulting and Clinical Psychology. 2004;72:371–381. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Lapitsky L, Seay S, Wallfisch A, Kitchens K. The effects of smoking schedules on cessation outcome: can we improve on common methods of gradual and abrupt nicotine withdrawal? Journal of Consulting and Clinical Psychology. 1995;63:388–399. doi: 10.1037//0022-006x.63.3.388. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Guyatt GH, Ryan G, Clifton J, Buckingham L, Willan A, et al. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA. 1993;269:2749–2753. [PubMed] [Google Scholar]
- Dalack GW, Meador-Woodruff JH. Acute feasibility and safety of a smoking reduction strategy for smokers with schizophrenia. Nicotine and Tobacco Research. 1999;1:53–57. doi: 10.1080/14622299050011151. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edn BMJ Publishing Group; London: 2001. [Google Scholar]
- Etter JF, Laszlo E, Perneger TV. Postintervention effect of nicotine replacement therapy on smoking reduction in smokers who are unwilling to quit: randomized trial. Journal of Clinical Psychopharmacology. 2004;24:174–179. doi: 10.1097/01.jcp.0000115666.45074.d6. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K-O. Smoking reduction in the management of COPD. Archives of Chest Diseases. 2002;57:281–284. [PubMed] [Google Scholar]
- Fagerstrom K-O. Clinical treatment of tobacco dependence: the endurance of pharmacologic efficacy. Journal of Clinical Psychiatry. 2003;18:35–40. [Google Scholar]
- Fagerstrom K-O, Hughes JR, Callas PW. Long-term effects of the Eclipse cigarette substitute and the nicotine inhaler in smokers not interested in quitting. Nicotine and Tobacco Research. 2002;2:S141–S145. doi: 10.1080/1462220021000032771. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K-O, Tejding R, Ake W, Lunell E. Aiding reduction of smoking with nicotine replacement medications: hope for the recalcitrant smoker? Tobacco Control. 1997;6:311–316. doi: 10.1136/tc.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falba T, Jofre-Bonet M, Busch S, Duchovny N, Sindelar J. Reduction of quantity smoked predicts future cessation among older smokers. Addiction. 2004;99:93–102. doi: 10.1111/j.1360-0443.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- Farkas AJ. When does cigarette fading increase the likelihood of future cessation? Annals of Behavioral Medicine. 1999;21:1–6. doi: 10.1007/BF02895036. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Treating Tobacco Use and Dependence Clinical Practice Guideline. US Public Health Service; Rockville, MD: 2000. [Google Scholar]
- Gajalakshmi CK, Jha P, Ranson K, Nguyen S. Global patterns of smoking and smoking-attributable mortality. In: Jha P, Chaloupka FJ, editors. Tobacco Control in Developing Countries. Oxford University Press; New York: 2000. pp. 11–39. [Google Scholar]
- George TP, Vessicchio JC, Termine A, Bregartner TA, Feingold A, Rounsaville BJ, et al. A placebo-controlled trial of bupropion for smoking cessation in schizophrenia. Biological Psychiatry. 2002;52:53–61. doi: 10.1016/s0006-3223(02)01339-2. [DOI] [PubMed] [Google Scholar]
- Gilpin EA, Pierce JP. The California tobacco control program and potential harm reduction through reduced cigarette consumption in continuing smokers. Nicotine and Tobacco Research. 2002;2:S157–S166. doi: 10.1080/1462220021000032708. [DOI] [PubMed] [Google Scholar]
- Giovino GA. Epidemiology of tobacco use in the UnitedStates. Oncogene. 2002;21:7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Klesges RC, Godding PR, Gegelman R. Controlled smoking, with or without carbon monoxide feedback, as an alternative for chronic smokers. Behavior Therapy. 1983;14:386–397. [Google Scholar]
- Glasgow RE, Klesges RC, Klesges LM, Vasey MW, Gunnarson DF. Long-term effects of a controlled smoking program: a 21/2 year follow-up. Behavior Therapy. 1985;16:303–307. [Google Scholar]
- Glasgow RE, Murray K, Lichtenstein E. Controlled smoking versus abstinence as a treatment goal: the hopes and fears may be unfounded. Behavior Therapy. 1989;20:77–91. [Google Scholar]
- Godtfredsen NS, Hoist C, Prescott E, Vestbo J, Osler M. Smoking reduction, smoking cessation, and mortality: a 16-year follow-up of 19,732 men and women from the Copenhagen Centre for Prospective Population Studies. American Journal of Epidemiology. 2002a;156:994–1001. doi: 10.1093/aje/kwf150. [DOI] [PubMed] [Google Scholar]
- Godtfredsen NS, Osler M, Vestbo J, Andersen I, Prescott E. Smoking reduction, smoking cessation, and incidence of fatal and non-fatal myocardial infarction in Denmark 1976–98: a pooled cohort study. Journal of Epidemiology and Community Health. 2003;57:412–416. doi: 10.1136/jech.57.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godtfredsen NS, Prescott E, Osler M, Vestbo J. Predictors of smoking reduction and cessation in a cohort of Danish moderate and heavy smokers. Preventive Medicine. 2001;33:46–52. doi: 10.1006/pmed.2001.0852. [DOI] [PubMed] [Google Scholar]
- Godtfredsen NS, Vestbo J, Osler M, Prescott E. Risk of hospital admission for COPD following smoking cessation and reduction: a Danish population study. Thorax. 2002b;57:967–972. doi: 10.1136/thorax.57.11.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Delucchi K, Velicer WF, Kahler CW, Ranger-Moore J, Hedeker D, et al. Statistical analysis of randomized trials in tobacco treatment: longitudinal designs with dichotomous outcome. Nicotine and Tobacco Research. 2001;3:193–203. doi: 10.1080/14622200110050411. [DOI] [PubMed] [Google Scholar]
- Hassmiller KM, Warner KE, Mendez D, Levy DT, Romano E. Nondaily smokers: who are they? American Journal of Public Health. 2003;93:1321–1327. doi: 10.2105/ajph.93.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Hecht SS, Hennrikus DJ, Joseph AM, Pentel PR. Biomarkers of tobacco exposure or harm:application to clinical and epidemiological studies. Nicotine and Tobacco Research. 2003;5:387–396. doi: 10.1080/1462220031000094222. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Rennard S, Patel MK, Kotlyar M, Malcolm R, Nides MA, et al. Effects of sustained-release bupropion among persons interested in reducing but not quitting smoking. American Journal of Medicine. 2004;116:151–157. doi: 10.1016/j.amjmed.2003.07.018. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Slade J, Benowitz NL, Giovino GA, Gritz ER, Leischow S, et al. Reducing tobacco harm:research challenges and issues. Nicotine and Tobacco Research. 2002;4:S89–S101. doi: 10.1080/1462220021000032852. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Murphy SE, Carmella SG, Zimmerman CL, Losey L, Kramarczuk I, et al. Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. Journal of the National Cancer Institute. 2004;96:107–115. doi: 10.1093/jnci/djh016. [DOI] [PubMed] [Google Scholar]
- Hennrikus DJ, Jeffery RW, Lando HA. Occasional smoking in a Minnesota working population. American Journal of Public Health. 1996;86:1260–1266. doi: 10.2105/ajph.86.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D, Weiss DJ, Walker DL, Jolley D. Long-term evaluation of controlled smoking as a treatment outcome. British Journal of Addiction. 1988;83:203–207. doi: 10.1111/j.1360-0443.1988.tb03982.x. [DOI] [PubMed] [Google Scholar]
- Ho-Yen DO, Spence VA, Moody JP, Walker WF. Why smoke fewer cigarettes? BMJ. 1982;284:1905–1907. doi: 10.1136/bmj.284.6333.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Reduced smoking: an introduction and review of the evidence. Addiction. 2000;95:S3–S7. doi: 10.1080/09652140032008. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Cummings KM, Hyland A. Ability of smokers to reduce their smoking and its association with future smoking cessation. Addiction. 1999;94:109–114. doi: 10.1046/j.1360-0443.1999.9411097.x. [DOI] [PubMed] [Google Scholar]
- Hughes GH, Hymowitz N, Ockene JK, Simon N, Vogt TM. The multiple risk factor intervention trial (MRFIT) Preventive Medicine. 1981;10:476–500. doi: 10.1016/0091-7435(81)90061-x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004a;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Lindgren PG, Connett JE, Nides MA. Reduction of smoking in the Lung Health Study. Nicotine and Tobacco Research. 2004b;6:275–280. doi: 10.1080/14622200410001676297. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Croghan GA, Wolter TD, Croghan IT, Offord KP, Williams GM, et al. Does smoking reduction result in reduction of biomarkers associated with harm? A pilot study using a nicotine inhaler. Nicotine and Tobacco Research. 2000;2:327–336. doi: 10.1080/713688154. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. Journal of Clinical Oncology. 2003;21:914–920. doi: 10.1200/JCO.2003.08.160. [DOI] [PubMed] [Google Scholar]
- Hyland A, Levy D, Rezaishiraz H, Hughes JR, Bauer JE, Giovino GA, et al. Nicotine and Tobacco Research. Reduction in amount smoked predicts future cessation. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . Clearing the Smoke Assessing the Science Base for Tobacco Harm Reduction. National Academy Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- Jimenez-Ruiz C, Solano S, Viteri SA, Ferrero MB, Torrecilla M, Mezuita MH. Harm reduction—a treatment approach for resistant smokers with tobacco-related symptoms. Respiration. 2002;69:452–455. doi: 10.1159/000064015. [DOI] [PubMed] [Google Scholar]
- Jolicoeur DG, Richter KP, Ahluwalia JS, Mosier MC, Resnicow K. Smoking cessation, smoking reduction, and delayed quitting among smokers given nicotine patches and a self-help pamphlet. Substance Abuse. 2003;24:101–106. doi: 10.1080/08897070309511538. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Bliss R, Zhao F, Lando H. Predictors of smoking reduction without formal intervention. Nicotine and Tobacco Research. 2005;7:277–282. doi: 10.1080/14622200500056176. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Strasser AA, Giovino GA, Erickson PA, Terza JV. Applying the risk/use equilibrium: use medicinal nicotine now for harm reduction. Tobacco Control. 2001;10:201–203. doi: 10.1136/tc.10.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando HA, Mcgovern PG. Three-year data on a behavioral treatment for smoking: a follow-up note. Addictive Behaviors. 1982;7:177–181. doi: 10.1016/0306-4603(82)90042-9. [DOI] [PubMed] [Google Scholar]
- Lichtenstein E, Rodrigues M-RP. Long-term effects of rapid smoking treatment for dependent cigarette smokers. Addictive Behaviors. 1977;2:109–112. doi: 10.1016/0306-4603(77)90027-2. [DOI] [PubMed] [Google Scholar]
- Lindstrom M, Isacsson SO. Smoking cessation among daily smokers aged 45–69 years: a longitudinal study in Malmö, Sweden. Addiction. 2002;97:205–215. doi: 10.1046/j.1360-0443.2002.00036.x. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention and treatment. Addictive Behaviors. 2002;27:867–886. doi: 10.1016/s0306-4603(02)00294-0. [DOI] [PubMed] [Google Scholar]
- McMorrow R, Fox RM. Nicotine’s role in smoking: an analysis of nicotine regulation. Psychological Bulletin. 1983;93:302–327. [PubMed] [Google Scholar]
- Meyer C, Rumpf HJ, Schumann A, Hapke U, Ulrich J. Intentionally reduced smoking among untreated general population smokers: prevalence, stability, prediction of smoking behaviour change and differences between subjects choosing either reduction or abstinence. Addiction. 2003;98:1101–1110. doi: 10.1046/j.1360-0443.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF.Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement Lancet 19993541896–1900.for the Quorom Group [DOI] [PubMed] [Google Scholar]
- National Cancer Institute . Proceedings of a Conference on What Works to Influence Cessation in the General Population, Smoking and Tobacco Control Monograph no. 12. US Public Health Service; Bethesda, MD: 2000. Population based smoking cessation. [Google Scholar]
- National Cancer Institute . Smoking and Tobacco Control Monograph no. 13. US Public Health Service; Bethesda, MD: 2001. Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine. [Google Scholar]
- Niaura R, Abrams DB. Smoking cessation: progress, priorities, and prospects. Journal of Consulting and Clinical Psychology. 2002;70:494–509. doi: 10.1037//0022-006x.70.3.494. [DOI] [PubMed] [Google Scholar]
- Norregaard J, Tonnesen P, Simonsen K, Petersen L, Sawe U. Smoking habits in relapsed subjects from a smoking cessation trial after one year. British Journal of Addiction. 1992;87:1189–1194. doi: 10.1111/j.1360-0443.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Chronic tolerance to nicotine in humans and its relationship to tobacco dependence. Nicotine and Tobacco Research. 2002;4:405–422. doi: 10.1080/1462220021000018425. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Baker TB. Any further progress in smoking cessation treatment? Nicotine and Tobacco Research. 2001;3:311–323. doi: 10.1080/14622200110050484. [DOI] [PubMed] [Google Scholar]
- Pisinger C, Vestbo J, Borch-Johnsen K, Jørgensen T. Smoking reduction intervention in a large population-based study. The Inter99 study. Preventive Medicine. 2005;40:112–118. doi: 10.1016/j.ypmed.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Porter S, Jackson K, Trosclair A, Pederson LL. Prevalence of current cigarette smoking among adults and changes in prevalence of current and some day smoking—United States, 1996–2001. Morbidity and Mortality Weekly Report. 2003;52:303–307. [PubMed] [Google Scholar]
- Rennard SI, Daughton D, Fujita J, Oehlerking MB, Dobson JR, Stahl MG, et al. Short-term smoking reduction is associated with reduction in measures of lower respiratory tract inflammation in heavy smokers. European Respiratory Journal. 1990;3:752–759. [PubMed] [Google Scholar]
- Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al. Systematic review of the effectiveness of stage based interventions to promote smoking cessation. BMJ. 2003;326:1–7. doi: 10.1136/bmj.326.7400.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs RL, Hughes JR, Pillitteri JL. Two behavioral treatments for smoking reduction: a pilot study. Nicotine and Tobacco Research. 2001;3:71–76. doi: 10.1080/14622200020032114. [DOI] [PubMed] [Google Scholar]
- Riley W, Jerome A, Behar A, Weil J. Computer and manual self-help behavioral strategies for smoking reduction:initial feasibility and one-year follow-up. Nicotine and Tobacco Research. 2002;2:S163–S168. doi: 10.1080/1462220021000032762. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Writing meta-analytic reviews. Psychological Bulletin. 1995;118:183–192. [Google Scholar]
- Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology. 1999;145:1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- Shadel WG, Shiffman S, Niaura R, Nichter M, Abrams DB. Current models of nicotine dependence: what is known and what is needed to advance understanding of tobacco etiology among youth. Drug and Alcohol Dependence. 2000;59:59–22. doi: 10.1016/s0376-8716(99)00162-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gitchell JG, Warner KE, Slade J, Henningfield JE, Pinney JM. Tobacco harm reduction: conceptual structure and nomenclature for analysis and research. Nicotine and Tobacco Research. 2002;4:S113–S129. doi: 10.1080/1462220021000032717. [DOI] [PubMed] [Google Scholar]
- Slade J, Henningfield JE. Tobacco product regulation: context and issues. In: Ogden JK, editor. Food and Drug Law Journal (Supplement) The Food and Drug Law Institute; Washington, DC: 1998. pp. 44–74. [PubMed] [Google Scholar]
- Spencer L, Pagell F, Hallion ME, Adams TB. Applying the transtheoretical model to tobacco cessation and prevention: a review of literature. American Journal of Health Promotion. 2002;17:7–71. doi: 10.4278/0890-1171-17.1.7. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stein JH, Bushara M, Bushara K, McCamish M, Jorenby DE, Fiore MC. Smoking cessation, but not smoking reduction, reduces plasma homocysteine levels. Clinical Cardiology. 2002;25:23–26. doi: 10.1002/clc.4950250107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser M. The tribulations of trials—intervention in communities. American Journal of Public Health. 2002;85:156–158. doi: 10.2105/ajph.85.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen P. Smoking cessation and smoking reduction in asthmatics. Nicotine and Tobacco Research. 2005;7:139–148. doi: 10.1080/14622200412331328411. [DOI] [PubMed] [Google Scholar]
- US Department Health and Human Services . A Report of the US Surgeon General. Office on Smoking and Health; Atlanta, GA: 1994. Preventing Tobacco Use Among Young People. [Google Scholar]
- US Department of Health and Human Services . A Report of the US Surgeon General. Office on Smoking and Health; Rockville, MD: 1990. The Health Benefits of Smoking Cessation. [Google Scholar]
- US Department of Health and Human Services . A Report of the US Surgeon General. Office of Smoking and Health; Atlanta, GA: 2000. Reducing Tobacco Use. [Google Scholar]
- US Department of Health and Human Services . A Report of the US Surgeon General. Office on Smoking and Health; Washington, DC: 2001. Women and Smoking. [Google Scholar]
- Warner KE. Tobacco harm reduction: promise and perils. Nicotine and Tobacco Research. 2003;3:S61–S71. doi: 10.1080/1462220021000032825. [DOI] [PubMed] [Google Scholar]
- Weiner E, Ball MP, Summerfelt A, Gold J, Buchanan RW. Effects of sustained-release bupropion and supportive group therapy on cigarette consumption in patients with schizophrenia. American Journal of Psychiatry. 2001;158:635–637. doi: 10.1176/appi.ajp.158.4.635. [DOI] [PubMed] [Google Scholar]
- Wennike P, Danielsson T, Landfeldt B, Westin A, Tonnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction. 2003;98:1395–1402. doi: 10.1046/j.1360-0443.2003.00489.x. [DOI] [PubMed] [Google Scholar]
- West R, McEwen A, Bolling K, Owen L. Smoking cessation and smoking patterns in the general population: a 1-year-follow-up. Addiction. 2001;96:891–902. doi: 10.1046/j.1360-0443.2001.96689110.x. [DOI] [PubMed] [Google Scholar]
- West R, Mcneill A, Raw M. Smoking cessation guidelines for health professionals: an update. Thorax. 2000;55:987–999. doi: 10.1136/thorax.55.12.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Welsch SK, Smith SS, Fouladi RT, Fiore MC, et al. Prevalence and predictors of transitions in smoking behavior among college students. Health Psychology. 2004;23:168–177. doi: 10.1037/0278-6133.23.2.168. [DOI] [PubMed] [Google Scholar]
- Wewers ME, Stillman FA, Hartmann AM, Shopland DR. Distribution of daily smokers by stage of change: current population survey results. Preventive Medicine. 2003;36:710–720. doi: 10.1016/s0091-7435(03)00044-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization . International Statistical Classification of Diseases and Related Problems, Tenth Revision. World Health Organization; Geneva: 1992. [Google Scholar]
- Zellweger JP. Anti-smoking therapies. Is harm reduction a viable alternative to smoking cessation? Drugs. 2001;61:1041–1044. doi: 10.2165/00003495-200161080-00001. [DOI] [PubMed] [Google Scholar]
- Zhu SH, Sun J, Hawkins S, Pierce J, Cummins S. A population study of low-rate smokers: quitting history and instability over time. Health Psychology. 2003;22:245–252. doi: 10.1037/0278-6133.22.3.245. [DOI] [PubMed] [Google Scholar]
APPENDIX
- Brue V, Lando H, Hendrickson J. A novel harm reduction technology for resistant smokers; Poster presented at the 8th Annual Meeting of the Society for Research an Nicotine and Tobacco; Savannah, GA, USA. February 2002.2002. [Google Scholar]
- Fornai E, Maggiorelli F, Pistelli F, Puntoni R, Desideri M, Molesti D, et al. Smoking cessation trial produces long-term reduction of cigarette consumption. European Respiratory Journal. 1996;9:170s. [Google Scholar]
- Gonzales D, Durcan M, Johnston A. Evaluation of bupropion sustained-release vs. placebo on number of cigarettes smoked by non-abstainers in placebo-controlled trials; Presented at the 6th Annual Meeting for the European Society for Research on Nicotine and Tobacco; Tubingen, Germany. October.2004. [Google Scholar]
- Haustein KO, Batra A, Landfeldt B, Westin A. The effect of short-term or long-term reduction on smoking cessation; results from a placebo controlled smoking reduction study with the nicotine gum. Nicotine and Tobacco Research. 2003;5:278. [Google Scholar]
- Joseph A, Hecht S, Murphy S, Gross M, Lando H, Bliss R, et al. A randomized controlled trial of smoking reduction in heart disease patients; Presented at the Annual Meeting of the Society for Research on Nicotine and Tobacco; Prague, Czech Republic. March.2004. [Google Scholar]
- Kotlyar M, Jensen J, Li S, Hatsukami D. Effect of smoking reduction on cardiovascular biomarkers and subjective measures; Paper Presented at the Annual Meeting of the Society for Research on Nicotine and Tobacco; Scottsdale, AZ. February.2004. [Google Scholar]
- Kralikova E, Kozak J, Rasmussen T. The clinical benefits of NRT-supported smoking reduction. Nicotine and Tobacco Research. 2002;4:243. [Google Scholar]
- Landfeldt B, Batra A, Friederich HM, Klingler K, Westin A. Smoking Reduction with a 4 mg Nicotine Gum—Final Results from a Placebo-Controlled Trial Over 13m; Presented at the Annual Meeting of the Society for Research on Nicotine and Tobacco; Europe, Padua, Italy. November.2003. [Google Scholar]
- Rennard SI, Daughton DM, Romberger DJ, Floreani AA, Robbins RA, Spurzem J, et al. Assessment of the pulmonary benefits of short-term cigarette reduction. Chest. 1992;102:6. [Google Scholar]
- Rennard SI, Daughton DM, Thompson AB, Floreani AA, Romberger DJ, Millatmal T. The effects of nicotine replacement therapy on cigarette smoking reduction; Paper Presented at the Annual Meeting of the American Thoracic Association; Boston, MA. May.1994. [Google Scholar]
- Rennard SI, Glover ED, Leischow S, Daughton DM, Glover P, Muramoto M, et al. Efficacy of the nicotine inhaler in smoking reduction. Nicotine and Tobacco Research. 2002;3:380. doi: 10.1080/14622200600789916. [DOI] [PubMed] [Google Scholar]
- Wileyto EP, Heitjan DF, Lerman C. Bupropion treatment does not reduce cigarette consumption for those who continue smoking; Paper Presented at the Annual Meeting of the. Society for Research on Nicotine and Tobacco; Scottsdale, AZ. February.2004. [Google Scholar]


