Abstract
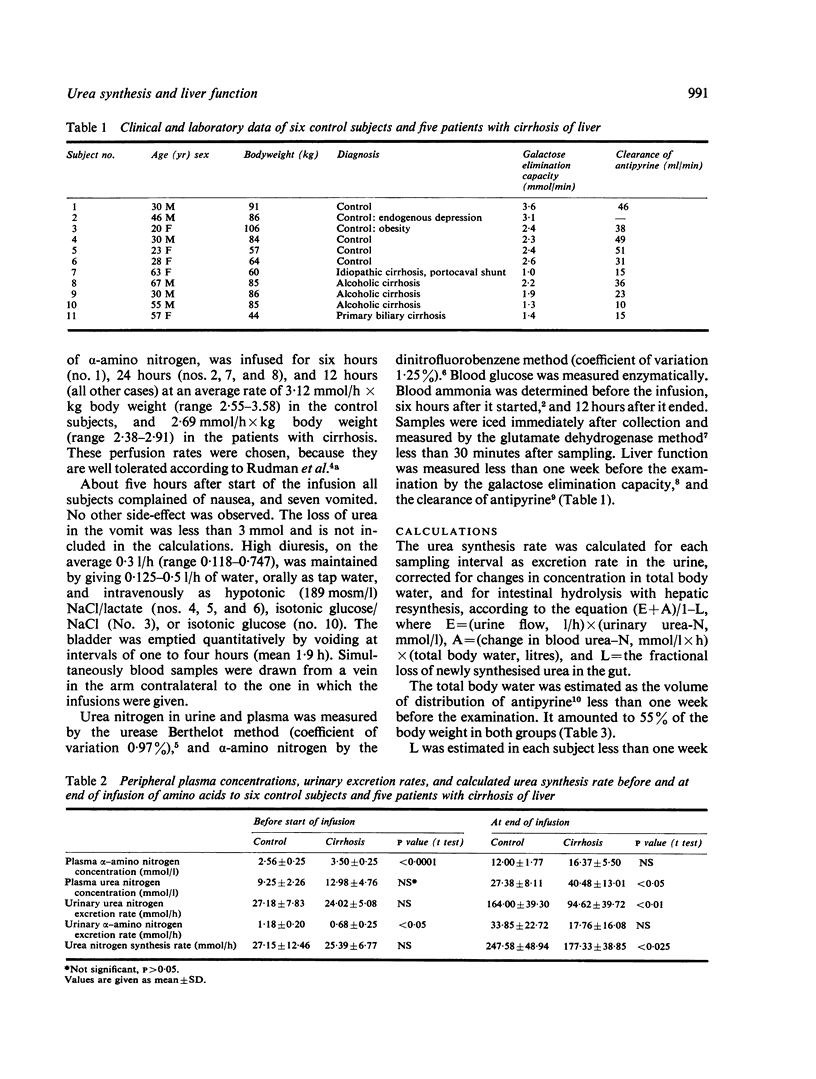
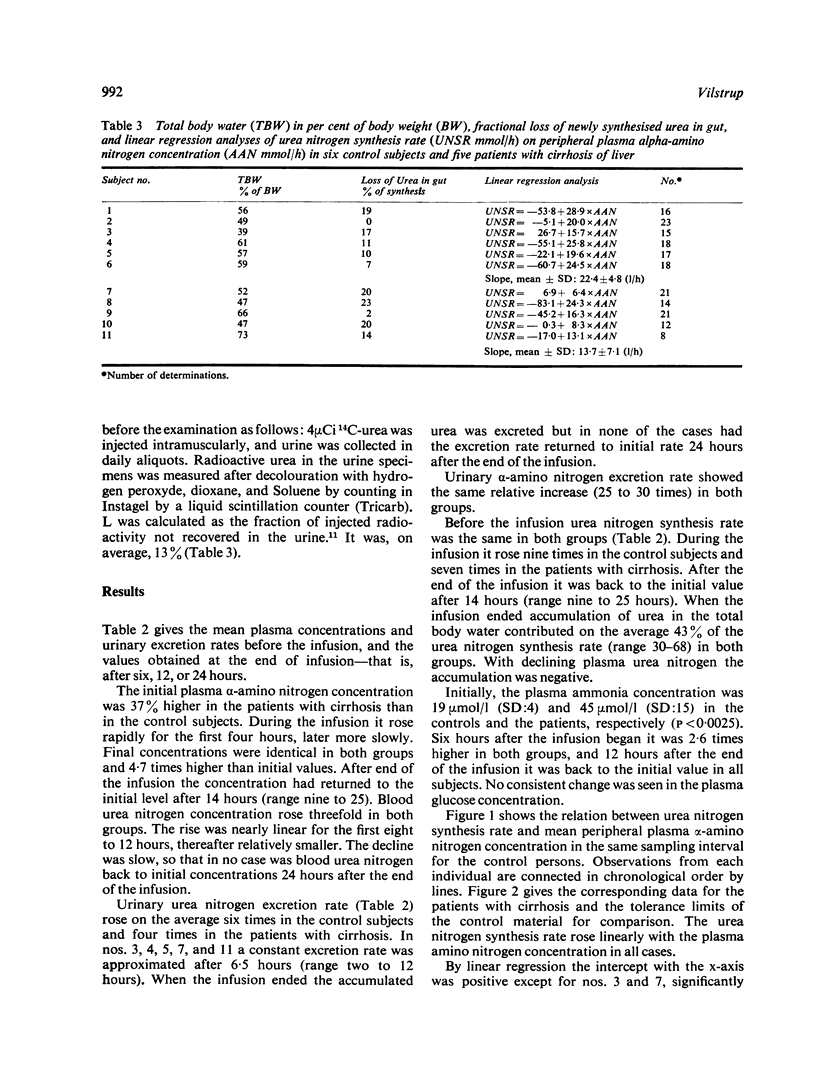
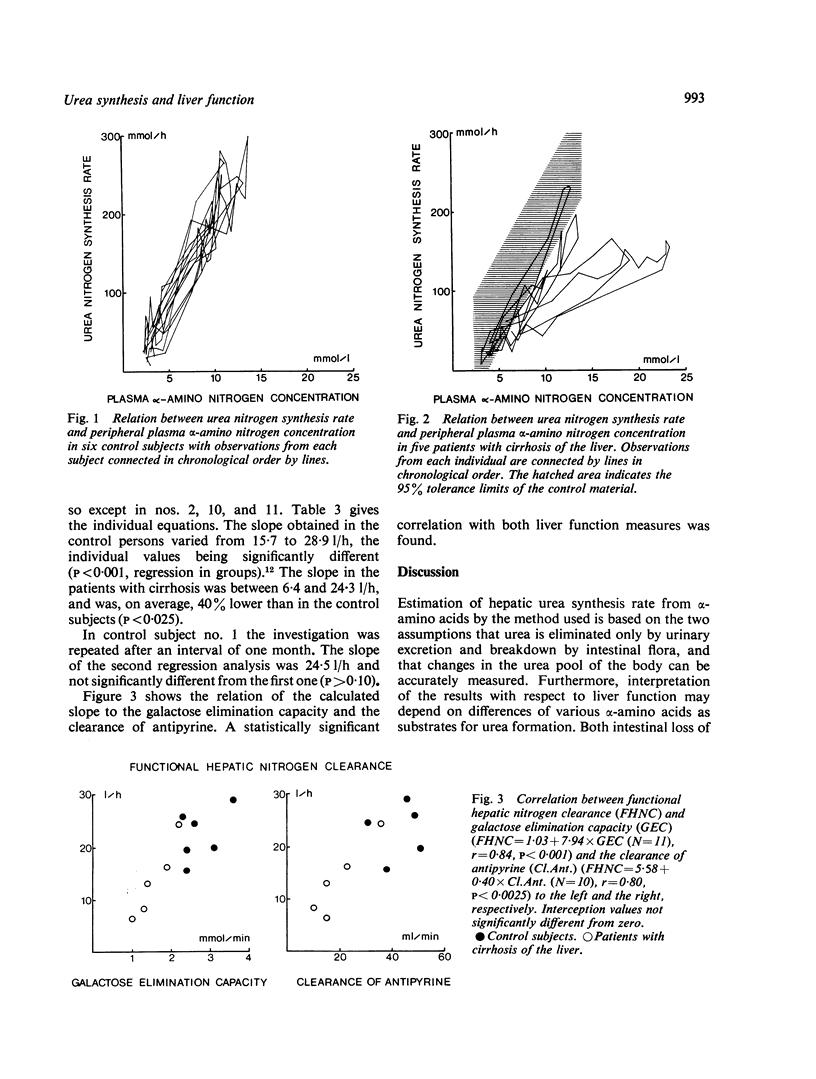
Hepatic urea synthesis is the organism's main channel for the disposal of nitrogen and it may be an 'essential' liver function. In six control subjects and five patients with cirrhosis of the liver urea synthesis was studied during continuous infusion for six to 24 hours of about 3 mmol alpha-amino nitrogen/h X kg body weight. The urea synthesis rate was calculated in intervals of two hours as urinary excretion with correction for accumulation in the total body water and for hydrolysis of urea in the gut. The peripheral venous plasma alpha-amino nitrogen concentration increased from 3 to about 14 mmol/l and the urea nitrogen synthesis rate from 25 to about 215 mmol/h. In all cases the urea synthesis rate rose linearly with the alpha-amino concentration throughout the examined range. The slope of this linear relationship is an expression of the hepatic conversion of alpha-amino nitrogen to urea nitrogen ('functional hepatic nitrogen clearance'). The functional hepatic nitrogen clearance was 22.4 l/h in control subjects and 13.7 1/h (P < 0.025) in the patients with cirrhosis. It was correlated with quantitative measures of the liver function (the galactose elimination capacity, r = 0.84, and the clearance of antipyrine, 4 = 0.80). These observations, while confirming the abundant capacity of the urea synthesis system, imply that a given urea synthesis rate requires a higher alpha-amino level in patients with reduced liver function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen P. B., Ranek L., Statland B. E., Tygstrup N. Clearance of antipyrine-dependence of quantitative liver function. Eur J Clin Invest. 1974 Apr;4(2):129–134. doi: 10.1111/j.1365-2362.1974.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Ansley J. D., Isaacs J. W., Rikkers L. F., Kutner M. H., Nordlinger B. M., Rudman D. Quantitative tests of nitrogen metabolism in cirrhosis: relation to other manifestations of liver disease. Gastroenterology. 1978 Oct;75(4):570–579. [PubMed] [Google Scholar]
- FAWCETT J. K., SCOTT J. E. A rapid and precise method for the determination of urea. J Clin Pathol. 1960 Mar;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos J. T., Warren W. D., Rudman D., Smith R. B., 3rd, Salam A. A. Selective and total shunts in the treatment of bleeding varices. A randomized controlled trial. N Engl J Med. 1976 Nov 11;295(20):1089–1095. doi: 10.1056/NEJM197611112952001. [DOI] [PubMed] [Google Scholar]
- Gibson J. A., Park N. J., Sladen G. E., Dawson A. M. The role of the colon in urea metabolism in man. Clin Sci Mol Med. 1976 Jan;50(1):51–59. doi: 10.1042/cs0500051. [DOI] [PubMed] [Google Scholar]
- Levin B. Hereditary metabolic disorders of the urea cycle. Adv Clin Chem. 1971;14:65–143. doi: 10.1016/s0065-2423(08)60145-6. [DOI] [PubMed] [Google Scholar]
- Long C. L., Jeevanandam M., Kinney J. M. Metabolism and recycling of urea in man. Am J Clin Nutr. 1978 Aug;31(8):1367–1382. doi: 10.1093/ajcn/31.8.1367. [DOI] [PubMed] [Google Scholar]
- Rafoth R. J., Onstad G. R. Urea synthesis after oral protein ingestion in man. J Clin Invest. 1975 Nov;56(5):1170–1174. doi: 10.1172/JCI108193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman D., DiFulco T. J., Galambos J. T., Smith R. B., 3rd, Salam A. A., Warren W. D. Maximal rates of excretion and synthesis of urea in normal and cirrhotic subjects. J Clin Invest. 1973 Sep;52(9):2241–2249. doi: 10.1172/JCI107410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypins E. B., Henderson J. M., Fulenwider J. T., Moffitt S., Galambos J. T., Warren W. D., Rudman D. A tracer method for measuring rate of urea synthesis in normal and cirrhotic subjects. Gastroenterology. 1980 Jun;78(6):1419–1424. [PubMed] [Google Scholar]
- Snodgrass P. J., Lin R. C., Müller W. A., Aoki T. T. Induction of urea cycle enzymes of rat liver by glucagon. J Biol Chem. 1978 Apr 25;253(8):2748–2753. [PubMed] [Google Scholar]
- Tygstrup N. Determination of the hepatic elimination capacity (Lm) of galactose by single injection. Scand J Clin Lab Invest Suppl. 1966;18:118–125. [PubMed] [Google Scholar]
- WALSER M., BODENLOS L. J. Urea metabolism in man. J Clin Invest. 1959 Sep;38:1617–1626. doi: 10.1172/JCI103940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS R. E., SMITH A. H., YOUNG G. A., PARSONS F. M., REED G. W. EXPERIMENTAL COMPARISON OF THE RATES AND VOLUMES OF DISTRIBUTION OF UREA, CREATININE, N-ACETYL-4-AMINOPHENAZONE, AND TRITIATED WATER. Br J Surg. 1964 Jul;51:544–549. doi: 10.1002/bjs.1800510721. [DOI] [PubMed] [Google Scholar]
- da Fonseca-Wollheim F. Bedeutung von Wasserstoffionenkonzentration und ADP-Zusatz bei der Ammoniakbestimmung mit Glutamatdehydrogenase. Verkesserter enzymatischer Ammoniaktest, I. Mitteilung. Z Klin Chem Klin Biochem. 1973 Oct;11(10):421–425. [PubMed] [Google Scholar]


