Abstract
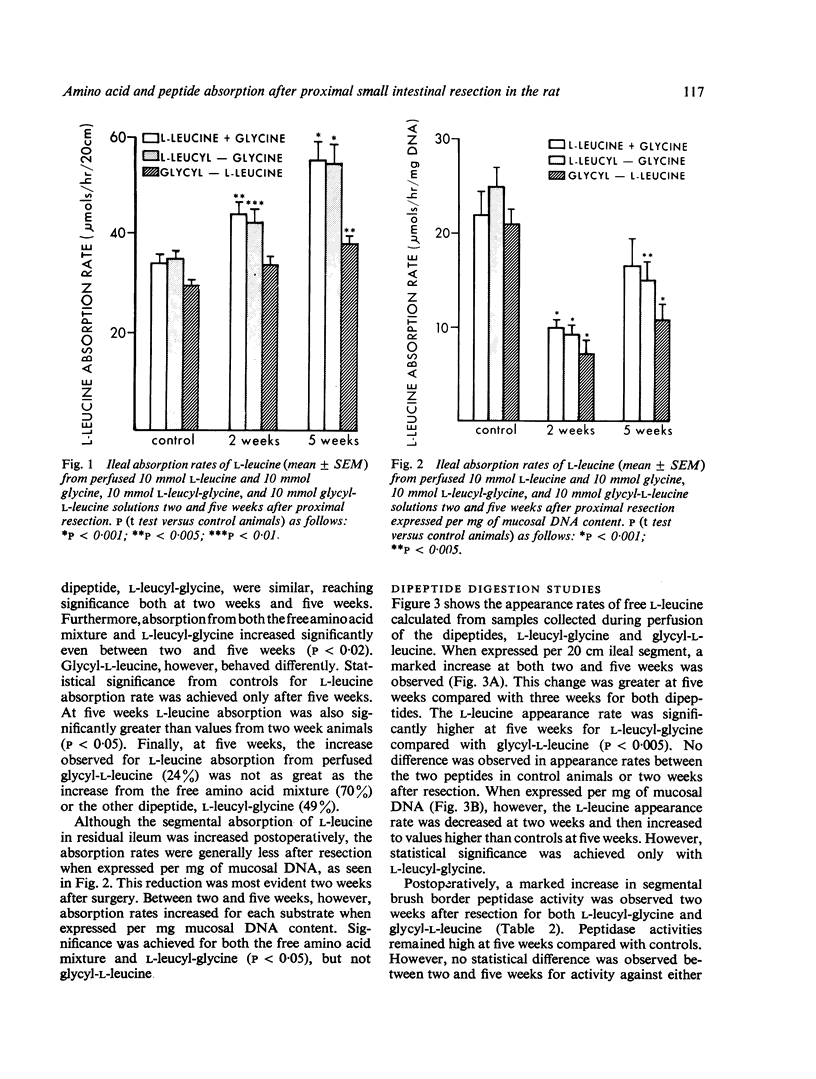
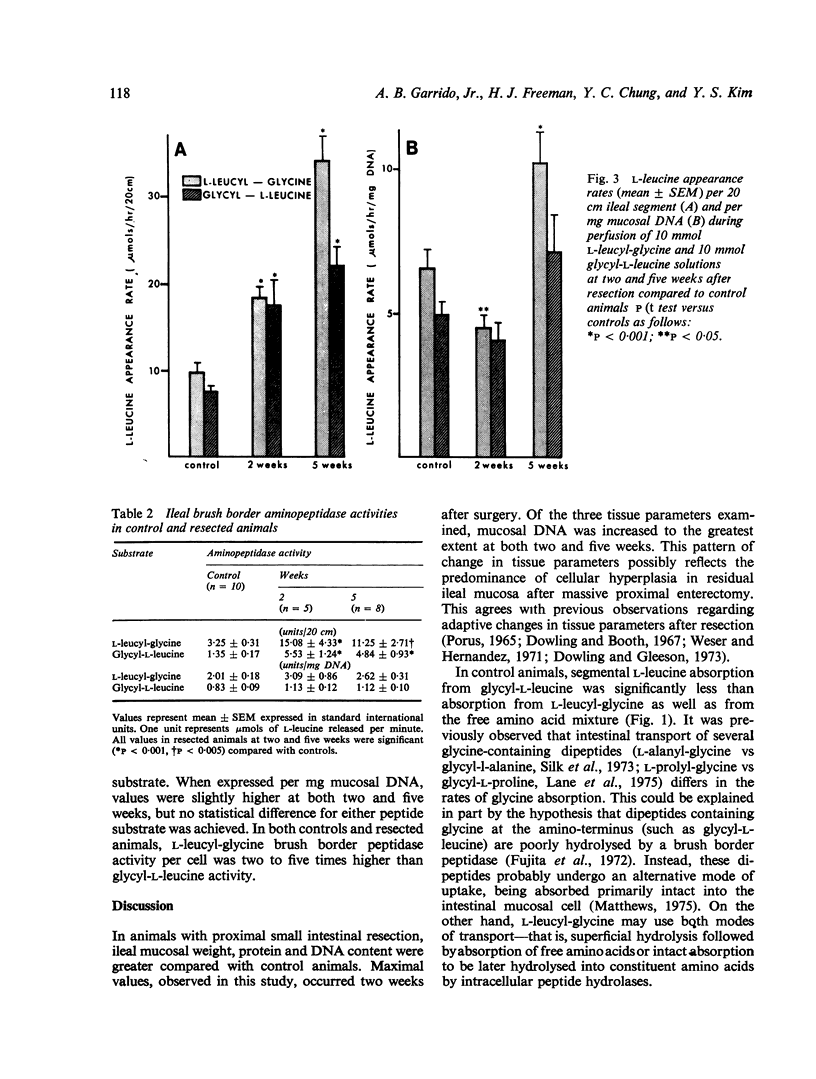
In experimental animals with massive proximal intestinal resection, in vivo ileal absorption of an amino acid mixture containing L-leucine and glycine as well as two different dipeptides, L-leucyl-glycine and glycyl-L-leucine, were compared. Both amino acid and dipeptide absorption were markedly enhanced in the ileal segments. However, the absorption rates from the two perfused dipeptides differed. L-leucine absorption from L-leucyl-glycine was much greater than from glycyl-L-leucine. Brush border amino-peptidase activities after resection were also increased but dissociation between absorption and hydrolytic activity occurred. This study indicates that certain dipeptides are handled differently by adapting ileal segments. Furthermore, the changes observed probably reflect mucosal cellular hyperplasia occurring in association with intestinal adaptation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A., Fogel M. R., Agrawal R. M. Comparison of free amino acid and dipeptide absorption in the jejunum of sprue patients. Gastroenterology. 1974 Oct;67(4):586–591. [PubMed] [Google Scholar]
- Adibi S. A. Intestinal phase of protein assimilation in man. Am J Clin Nutr. 1976 Feb;29(2):205–215. doi: 10.1093/ajcn/29.2.205. [DOI] [PubMed] [Google Scholar]
- Craft I. L., Geddes D., Hyde C. W., Wise I. J., Matthews D. M. Absorption and malabsorption of glycine and glycine peptides in man. Gut. 1968 Aug;9(4):425–437. doi: 10.1136/gut.9.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Dowling R. H., Gleeson M. H. Cell turnover following small bowel resection and by-pass. Digestion. 1973;8(2):176–190. doi: 10.1159/000197312. [DOI] [PubMed] [Google Scholar]
- Fujita M., Parsons D. S., Wojnarowska F. Oligopeptidases of brush border membranes of rat small intestinal mucosal cells. J Physiol. 1972 Dec;227(2):377–394. doi: 10.1113/jphysiol.1972.sp010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M. H., Dowling R. H., Peters T. J. Biochemical changes in intestinal mucosa after experimental small bowel by-pass in the rat. Clin Sci. 1972 Dec;43(6):743–757. doi: 10.1042/cs0430743. [DOI] [PubMed] [Google Scholar]
- Heizer W. D., Kerley R. L., Isselbacher K. J. Intestinal peptide hydrolases differences between brush border and cytoplasmic enzymes. Biochim Biophys Acta. 1972 May 16;264(3):450–461. doi: 10.1016/0304-4165(72)90008-6. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kim Y. S., McCarthy D. M., Lane W., Fong W. Alterations in the levels of peptide hydrolases and other enzymes in brush-border and soluble fractions of rat small intestinal mucosa during starvation and refeeding. Biochim Biophys Acta. 1973 Sep 15;321(1):262–273. doi: 10.1016/0005-2744(73)90081-8. [DOI] [PubMed] [Google Scholar]
- LORAN M. R., ALTHAUSEN T. L. Transport of vitamin A in vitro across normal isolated rat intestine and intestine subjected to 'partial' resection. Am J Physiol. 1959 Dec;197:1333–1336. doi: 10.1152/ajplegacy.1959.197.6.1333. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane A. E., Silk D. B., Clark M. L. Absorption of two proline containing peptides by rat small intestine in vivo. J Physiol. 1975 Jun;248(1):143–149. doi: 10.1113/jphysiol.1975.sp010966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews D. M., Adibi S. A. Peptide absorption. Gastroenterology. 1976 Jul;71(1):151–161. [PubMed] [Google Scholar]
- Matthews D. M. Intestinal absorption of peptides. Physiol Rev. 1975 Oct;55(4):537–608. doi: 10.1152/physrev.1975.55.4.537. [DOI] [PubMed] [Google Scholar]
- Matthews D. M. Introduction. Membrane transport of peptides. Ciba Found Symp. 1977;(50):5–14. [PubMed] [Google Scholar]
- McCarthy D. M., Kim Y. S. Changes in sucrase, enterokinase, and peptide hydrolase after intestinal resection. The association of cellular hyperplasia and adaptation. J Clin Invest. 1973 Apr;52(4):942–951. doi: 10.1172/JCI107259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard K. Resection of the small intestine in rats. 3. Morphological changes in the intestinal tract. Acta Chir Scand. 1967;133(3):233–248. [PubMed] [Google Scholar]
- PORUS R. L. EPITHELIAL HYPERPLASIA FOLLOWING MASSIVE SMALL BOWEL RESECTION IN MAN. Gastroenterology. 1965 Jun;48:753–757. [PubMed] [Google Scholar]
- Silk D. B. Amino acid and peptide absorption in man. Ciba Found Symp. 1977;(50):15–29. doi: 10.1002/9780470720318.ch3. [DOI] [PubMed] [Google Scholar]
- Silk D. B., Kumar P. J., Perrett D., Clark M. L., Dawson A. M. Amino acid and peptide absorption in patients with coeliac disease and dermatitis herpetiformis. Gut. 1974 Jan;15(1):1–8. doi: 10.1136/gut.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk D. B., Nicholson J. A., Kim Y. S. Relationships between mucosal hydrolysis and transport of two phenylalanine dipeptides. Gut. 1976 Nov;17(11):870–876. doi: 10.1136/gut.17.11.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk D. B., Perrett D., Clark M. L. Intestinal transport of two dipeptides containing the same two neutral amino acids in man. Clin Sci Mol Med. 1973 Sep;45(3):291–299. doi: 10.1042/cs0450291. [DOI] [PubMed] [Google Scholar]
- Silk D. B., Perrett D., Webb J. P., Clark M. L. Absorption of two tripeptides by the human small intestine: a study using a perfusion technique. Clin Sci Mol Med. 1974 Mar;46(3):393–402. doi: 10.1042/cs0460393. [DOI] [PubMed] [Google Scholar]
- Silk D. B. Progress report. Peptide absorption in man. Gut. 1974 Jun;15(6):494–501. doi: 10.1136/gut.15.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. R., Stephens R. V., Randall H. T., Bowen J. R. Use of the "space diet" in the management of a patient with extreme short bowel syndrome. Am J Surg. 1969 Apr;117(4):449–459. doi: 10.1016/0002-9610(69)90003-8. [DOI] [PubMed] [Google Scholar]
- Weser E., Hernandez M. H. Studies of small bowel adaptation after intestinal resection in the rat. Gastroenterology. 1971 Jan;60(1):69–75. [PubMed] [Google Scholar]


