Abstract
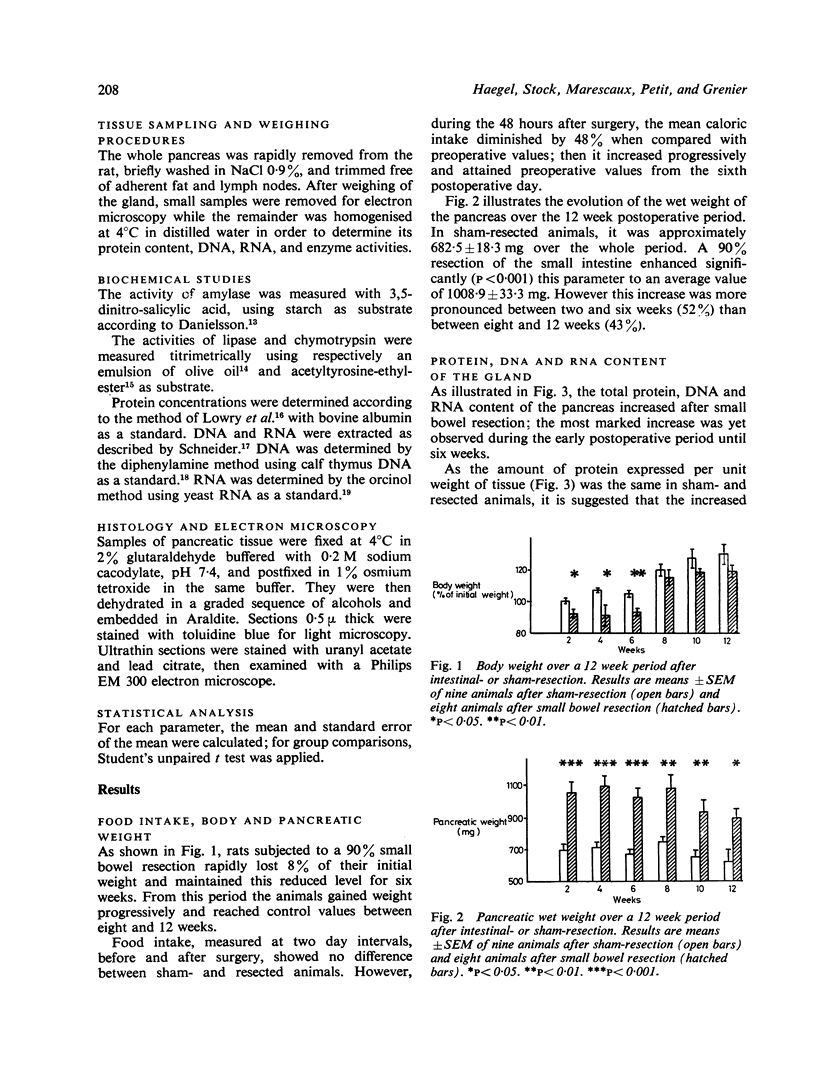
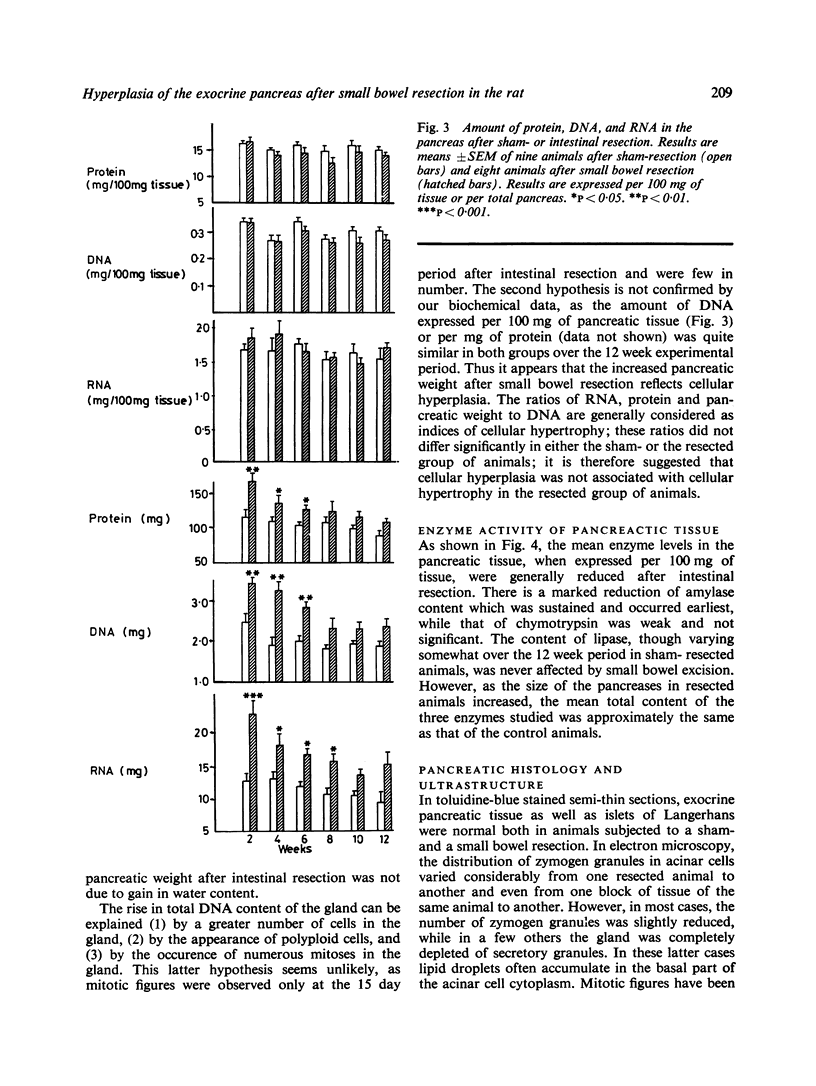
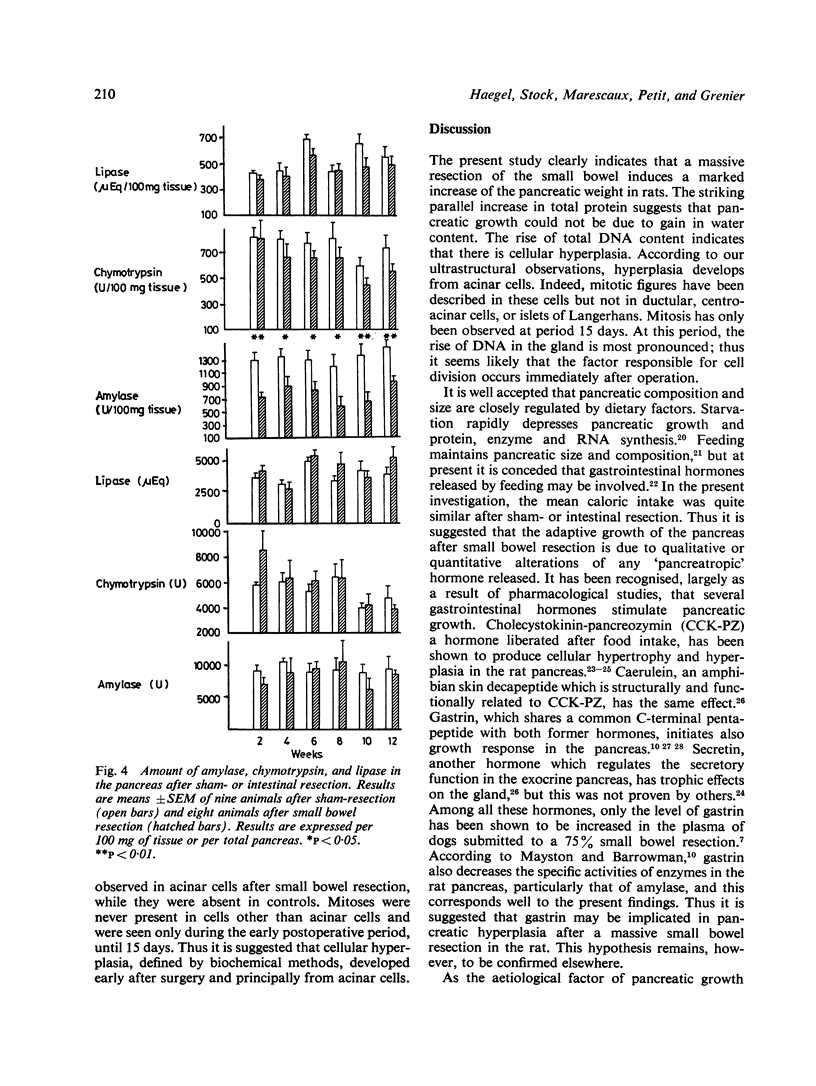
The effect of a 90% small bowel resection on the exocrine pancreas was investigated over a three month period in adult Wistar rats. Control animals underwent a sham-resection consisting of a transection and reanastomosis of the small intestine. After jejunoileal resection, the wet weight of a gland increased significantly (52%) from the 15th day. The parallel increase in total protein, DNA and RNA content without any modification in the ratios of pancreatic weight, protein, and RNA to DNA suggests that there is cellular hyperplasia but not hypertrophy. Small intestinal resection decreased significantly the amount of amylase when expressed per unit pancreatic weight; it reduced slightly but not significantly that of chymotrypsin, while it did not modify the amount of lipase. However, the total amount of these enzymes in the pancreas remained unaltered when compared with controls. It is concluded that a massive resection of the small bowel induces cellular hyperplasia in the rat exocrine pancreas; this could compensate that reduced level of enzymes in acinar cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G. G. Influence of bile and pancreatic secretions on the size of the intestinal villi in the rat. Am J Anat. 1971 Oct;132(2):167–177. doi: 10.1002/aja.1001320204. [DOI] [PubMed] [Google Scholar]
- Brants F., Morisset J. Tropic effect of cholecystokinin-pancreozymin on pancreatic acinar cells from rats of different ages. Proc Soc Exp Biol Med. 1976 Dec;153(3):523–527. doi: 10.3181/00379727-153-39583. [DOI] [PubMed] [Google Scholar]
- Buxton B. Small bowel resection and gastric acid hypersecretion. Gut. 1974 Mar;15(3):229–238. doi: 10.1136/gut.15.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'sa A. A., Buchanan K. D. Role of gastrointestinal hormones in the response to massive resection of the small bowel. Gut. 1977 Nov;18(11):877–881. doi: 10.1136/gut.18.11.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson A. Techniques for measuring amylase secretion from pieces of mouse pancreas. Anal Biochem. 1974 May;59(1):220–234. doi: 10.1016/0003-2697(74)90028-1. [DOI] [PubMed] [Google Scholar]
- Dembinski A. B., Johnson L. R. Growth of pancreas and gastrointestinal mucosa in antrectomized and gastrin-treated rats. Endocrinology. 1979 Sep;105(3):769–773. doi: 10.1210/endo-105-3-769. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Fölsch U. R., Winckler K., Wormsley K. G. Influence of repeated administration of cholecystokinin and secretin on the pancreas of the rat. Scand J Gastroenterol. 1978;13(6):663–671. doi: 10.3109/00365527809181779. [DOI] [PubMed] [Google Scholar]
- Hanson W. R., Osborne J. W., Sharp J. G. Compensation by the residual intestine after intestinal resection in the rat. II. Influence of postoperative time interval. Gastroenterology. 1977 Apr;72(4 Pt 1):701–705. [PubMed] [Google Scholar]
- Johnson L. R., Copeland E. M., Dudrick S. J., Lichtenberger L. M., Castro G. A. Structural and hormonal alterations in the gastrointestinal tract of parenterally fed rats. Gastroenterology. 1975 May;68(5 Pt 1):1177–1183. [PubMed] [Google Scholar]
- Johnson L. R. The trophic action of gastrointestinal hormones. Gastroenterology. 1976 Feb;70(2):278–288. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARCHIS-MOUREN G., SARDA L., DESNUELLE P. Purification of hog pancreatic lipase. Arch Biochem Biophys. 1959 Jul;83(1):309–319. doi: 10.1016/0003-9861(59)90036-0. [DOI] [PubMed] [Google Scholar]
- Mainz D. L., Black O., Webster P. D. Hormonal control of pancreatic growth. J Clin Invest. 1973 Sep;52(9):2300–2304. doi: 10.1172/JCI107418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayston P. D., Barrowman J. A. Influence of chronic administration of pentagastrin on the pancreas in hypophysectomized rats. Gastroenterology. 1973 Mar;64(3):391–399. [PubMed] [Google Scholar]
- Mayston P. D., Barrowman J. A. The influence of chronic administration of pentagastrin on the rat pancreas. Q J Exp Physiol Cogn Med Sci. 1971 Apr;56(2):113–122. doi: 10.1113/expphysiol.1971.sp002105. [DOI] [PubMed] [Google Scholar]
- McDermott F. T., Roudnew B. Ileal crypt cell population kinetics after 40% small bowel resection. Autoradiographic studies in the rat. Gastroenterology. 1976 May;70(5 PT1):707–711. [PubMed] [Google Scholar]
- Morisset J. A., Black O., Jr, Webster P. D. Effects of fasting, feeding, and bethanechol chloride on pancreatic microsomal protein synthesis in vitro. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1308–1314. doi: 10.3181/00379727-140-36664. [DOI] [PubMed] [Google Scholar]
- Oscarson J. E., Veen H. F., Williamson R. C., Ross J. S., Malt R. A. Compensatory postresectional hyperplasia and starvation atrophy in small bowel: dissociation from endogenous gastrin levels. Gastroenterology. 1977 May;72(5 Pt 1):890–895. [PubMed] [Google Scholar]
- REBOUD J. P., BEN ABDEL JLIL A., DESNUELLE P. [Variations in the enzyme content of the rat pancreas as a function of the composition of the diet]. Biochim Biophys Acta. 1962 Apr 9;58:326–337. doi: 10.1016/0006-3002(62)91016-8. [DOI] [PubMed] [Google Scholar]
- Reber H. A., Johnson F., Deveney K., Montgomery C., Way L. W. Trophic effects of gastrin on the exocrine pancreas in rats. J Surg Res. 1977 May;22(5):554–560. doi: 10.1016/0022-4804(77)90040-3. [DOI] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Solomon T. E., Petersen H., Elashoff J., Grossman M. I. Interaction of caerulein and secretin on pancreatic size and composition in rat. Am J Physiol. 1978 Dec;235(6):E714–E719. doi: 10.1152/ajpendo.1978.235.6.E714. [DOI] [PubMed] [Google Scholar]
- Webster P. D., Singh M., Tucker P. C., Black O. Effects of fasting and feeding on the pancreas. Gastroenterology. 1972 Apr;62(4):600–605. [PubMed] [Google Scholar]
- Weser E., Heller R., Tawil T. Stimulation of mucosal growth in the rat ileum by bile and pancreatic secretions after jejunal resection. Gastroenterology. 1977 Sep;73(3):524–529. [PubMed] [Google Scholar]
- Weser E., Hernandez M. H. Studies of small bowel adaptation after intestinal resection in the rat. Gastroenterology. 1971 Jan;60(1):69–75. [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L., Ross J. S., Malt R. A. Contributions of bile and pancreatic juice to cell proliferation in ileal mucosa. Surgery. 1978 May;83(5):570–576. [PubMed] [Google Scholar]
- Williamson R. C. Intestinal adaptation (second of two parts). Mechanisms of control. N Engl J Med. 1978 Jun 29;298(26):1444–1450. doi: 10.1056/NEJM197806292982604. [DOI] [PubMed] [Google Scholar]
- Winborn W. B., Seelig L. L., Jr, Nakayama H., Weser E. Hyperplasia of the gastric glands after small bowel resection in the rat. Gastroenterology. 1974 Mar;66(3):384–395. [PubMed] [Google Scholar]


