Abstract
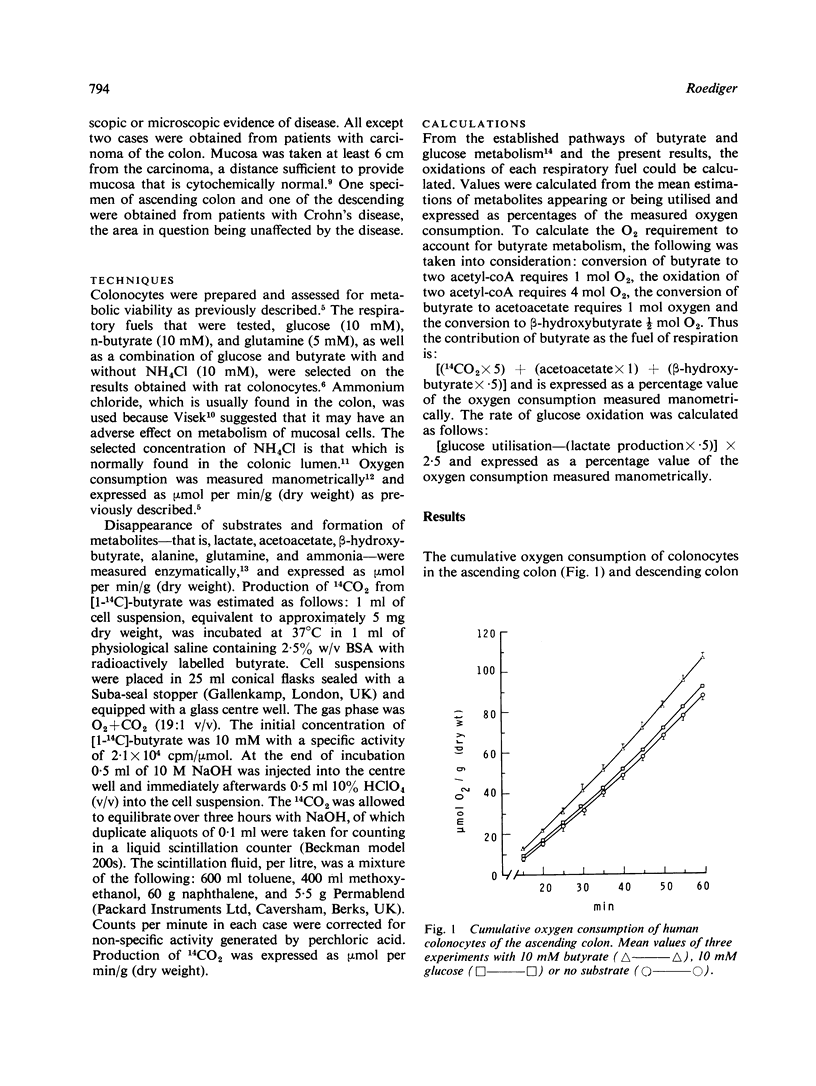
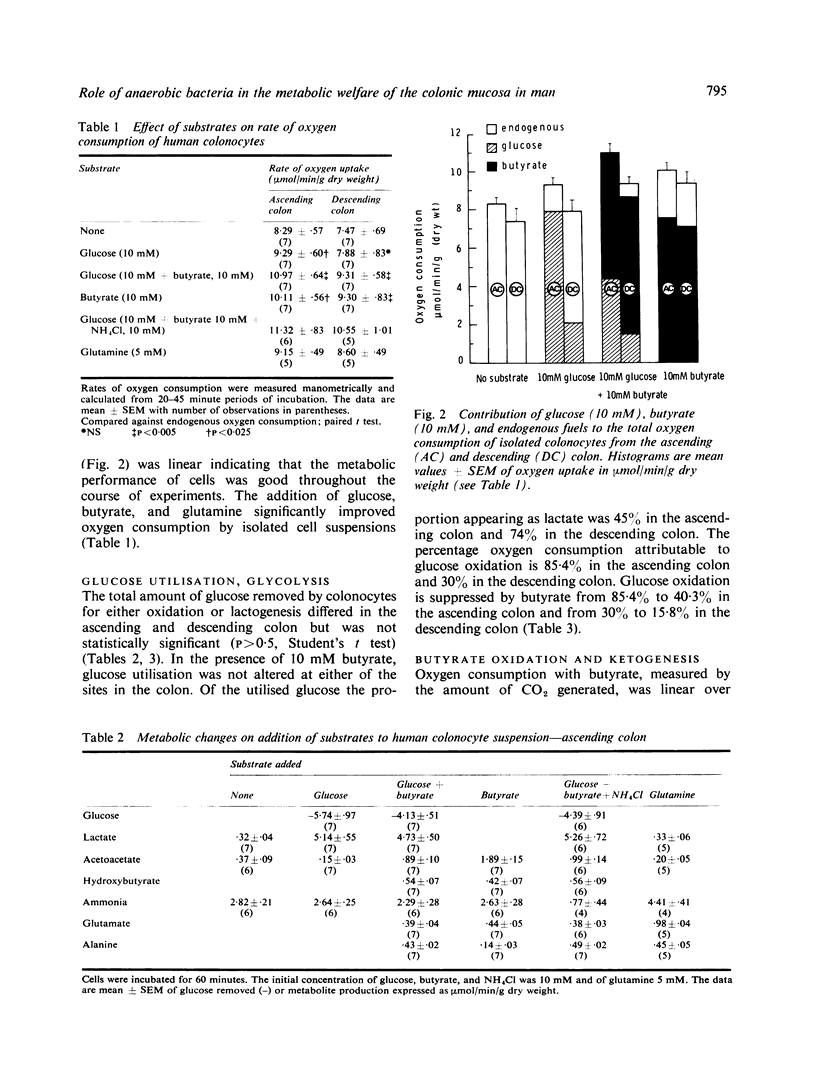
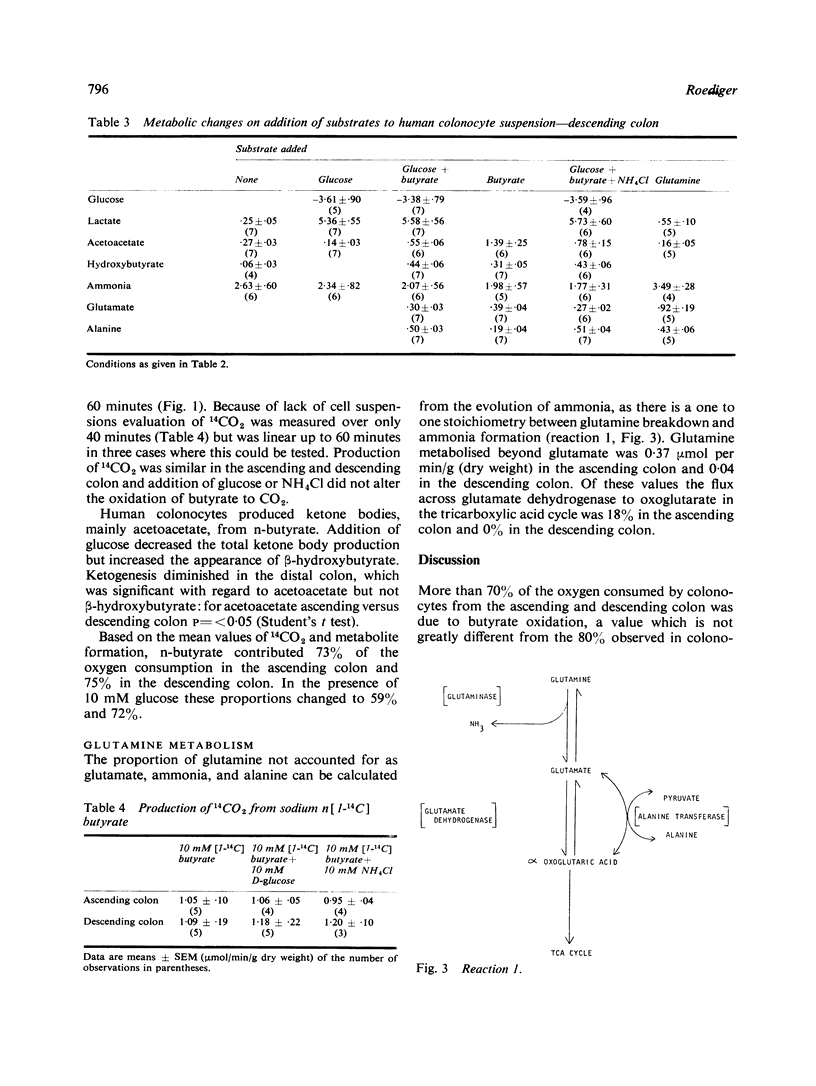
Suspensions of isolated epithelial cells (colonocytes) from the human colon were used to assess utilisation of respiratory fuels which are normally available to the colonic mucosa in vivo. Cells were prepared from operative specimens of the ascending colon (seven) and descending colon (seven). The fuels that were used were the short chain fatty acid n-butyrate, produced only by anaerobic bacteria in the colonic lumen, together with glucose and glutamine, normally present in the circulation. The percentage oxygen consumption attributable to n-butyrate, when this was the only substrate, was 73% in the ascending colon and 75% in the descending colon. In the presence of 10 mM glucose these proportions changed to 59% and 72%. Aerobic glycolysis was observed in both the ascending and descending colon. Glucose oxidation accounted for 85% of the oxygen consumption in the ascending colon and 30% in the descending colon. In the presence of 10 mM n-butyrate these proportions decreased to 41% in the ascending colon and 16% in the descending colon. Based on the assumption that events in the isolated colonocytes reflect utilization of fuels in vivo, the hypothesis is put forward that fatty acids of anaerobic bacteria are a major source of energy for the colonic mucosa, particularly of the distal colon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Filipe M. I., Branfoot A. C. Abnormal patterns of mucus secretion in apparently normal mucosa of large intestine with carcinoma. Cancer. 1974 Aug;34(2):282–290. doi: 10.1002/1097-0142(197408)34:2<282::aid-cncr2820340211>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hanson P. J., Parsons S. Metabolism and transport of glutamine and glucose in vascularly perfused small intestine rat. Biochem J. 1977 Sep 15;166(3):509–519. doi: 10.1042/bj1660509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning S. J., Hird F. J. Ketogenesis from butyrate and acetate by the caecum and the colon of rabbits. Biochem J. 1972 Dec;130(3):785–790. doi: 10.1042/bj1300785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972;8:1–34. [PubMed] [Google Scholar]
- McNeil N. I., Cummings J. H., James W. P. Short chain fatty acid absorption by the human large intestine. Gut. 1978 Sep;19(9):819–822. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus L. M., Windmueller H. G. Phosphate-dependent glutaminase of small intestine: localization and role in intestinal glutamine metabolism. Arch Biochem Biophys. 1977 Aug;182(2):506–517. doi: 10.1016/0003-9861(77)90531-8. [DOI] [PubMed] [Google Scholar]
- Roediger W. E., Truelove S. C. Method of preparing isolated colonic epithelial cells (colonocytes) for metabolic studies. Gut. 1979 Jun;20(6):484–488. doi: 10.1136/gut.20.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein R., Howard A. V., Wrong O. M. In vivo dialysis of faeces as a method of stool analysis. IV. The organic anion component. Clin Sci. 1969 Oct;37(2):549–564. [PubMed] [Google Scholar]
- Schmitt M. G., Jr, Soergel K. H., Wood C. M., Steff J. J. Absorption of short-chain fatty acids from the human ileum. Am J Dig Dis. 1977 Apr;22(4):340–347. doi: 10.1007/BF01072192. [DOI] [PubMed] [Google Scholar]
- Stevens C. E. Physiological implications of microbial digestion in the large intestine of mammals: relation to dietary factors. Am J Clin Nutr. 1978 Oct;31(10 Suppl):S161–S168. doi: 10.1093/ajcn/31.10.S161. [DOI] [PubMed] [Google Scholar]
- Vercellotti J. R., Salyers A. A., Bullard W. S., Wilkins D. Breakdown of mucin and plant polysaccharides in the human colon. Can J Biochem. 1977 Nov;55(11):1190–1196. doi: 10.1139/o77-178. [DOI] [PubMed] [Google Scholar]
- Watford M., Lund P., Krebs H. A. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J. 1979 Mar 15;178(3):589–596. doi: 10.1042/bj1780589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J Biol Chem. 1978 Jan 10;253(1):69–76. [PubMed] [Google Scholar]


