Abstract
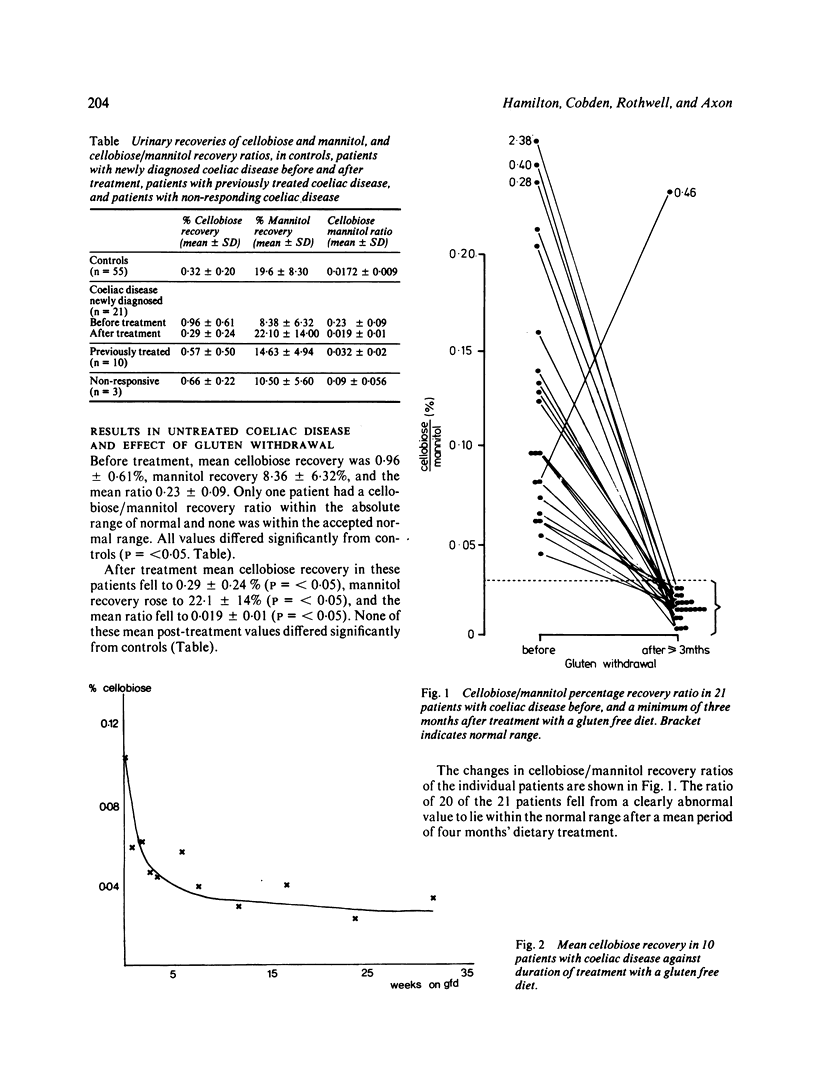
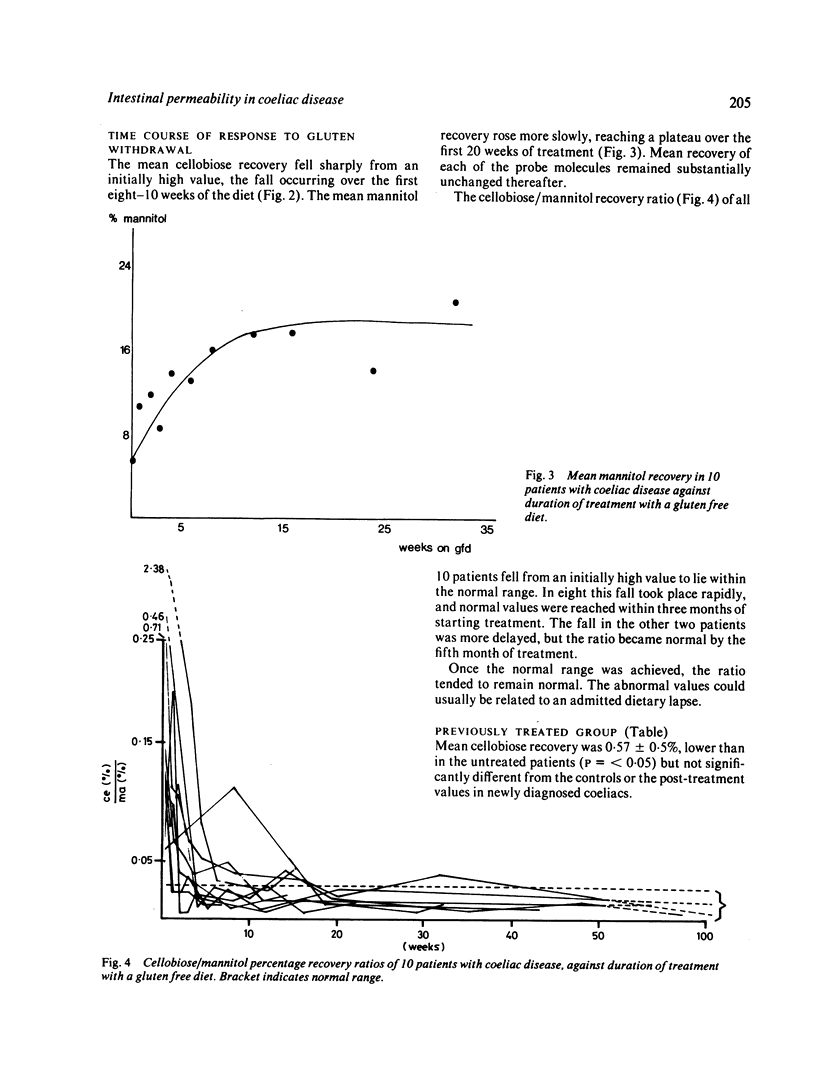
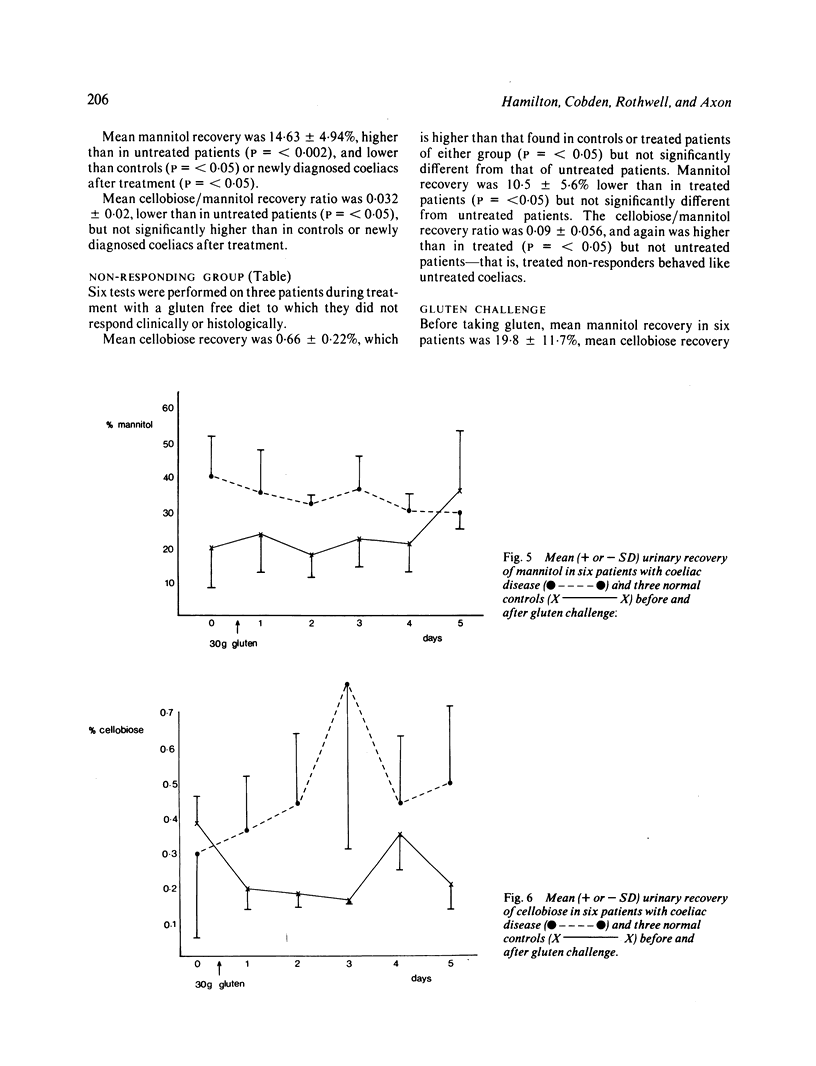
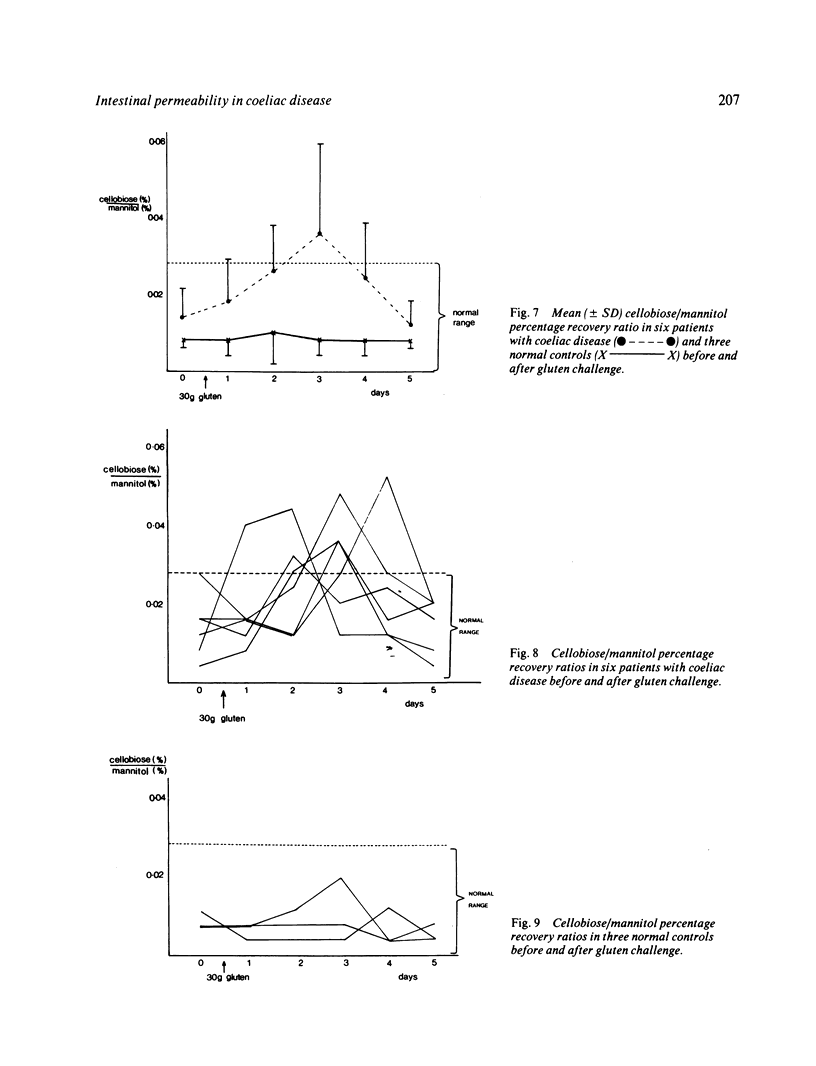
Intestinal permeability has been studied in 21 patients with coeliac disease in relapse and after gluten withdrawal using an oral test of intestinal permeability based on the simultaneous oral administration of two probe molecules. The increased absorption of the larger molecule (cellobiose) and the decreased absorption of the smaller (mannitol) found in untreated coeliac disease both returned to normal within five months of starting treatment, the abnormality in cellobiose absorption correcting more rapidly than that of mannitol. After exposure to a single oral dose of gluten, the intestinal permeability of six patients with treated coeliac disease became transiently abnormal with an increased absorption of cellobiose, returning to normal within one week. The possible structural and functional implications of these findings are discussed. The cellobiose/mannitol ratio appears to be of value in assessing the response to gluten withdrawal in coeliac disease, and also in monitoring patients who are already established on a gluten free diet by detecting dietary lapses and 'non-responding coeliac disease'. It may also offer an alternative to jejunal biopsy in patients subjected to gluten challenge.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado F. D-xylose active transport in the hamster small intestine. Biochim Biophys Acta. 1966 Feb 7;112(2):292–306. doi: 10.1016/0926-6585(66)90328-1. [DOI] [PubMed] [Google Scholar]
- Chadwick V. S., Phillips S. F., Hofmann A. F. Measurements of intestinal permeability using low molecular weight polyethylene glycols (PEG 400). I. Chemical analysis and biological properties of PEG 400. Gastroenterology. 1977 Aug;73(2):241–246. [PubMed] [Google Scholar]
- Chadwick V. S., Phillips S. F., Hofmann A. F. Measurements of intestinal permeability using low molecular weight polyethylene glycols (PEG 400). II. Application to normal and abnormal permeability states in man and animals. Gastroenterology. 1977 Aug;73(2):247–251. [PubMed] [Google Scholar]
- Cobden I., Dickinson R. J., Rothwell J., Axon A. T. Intestinal permeability assessed by excretion ratios of two molecules: results in coeliac disease. Br Med J. 1978 Oct 14;2(6144):1060–1060. doi: 10.1136/bmj.2.6144.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobden I., Rothwell J., Axon A. T. Intestinal permeability and screening tests for coeliac disease. Gut. 1980 Jun;21(6):512–518. doi: 10.1136/gut.21.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobden I., Rothwell J., Axon A. T. Intestinal permeability in rats infected by Nippostrongylus brasiliensis. Gut. 1979 Aug;20(8):716–721. doi: 10.1136/gut.20.8.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobden I., Rothwell J., Axon A. T. Non-invasive test for small-intestinal mucosal damage. Lancet. 1979 Dec 22;2(8156-8157):1379–1379. doi: 10.1016/s0140-6736(79)92871-x. [DOI] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Jr, Ewton M. F., Soter N., Kinney J. Permeability characteristics of the human small intestine. J Clin Invest. 1965 Dec;44(12):1935–1944. doi: 10.1172/JCI105299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Locklear T. W., Ewton M. F. Water and solute movement in the small intestine of patients with sprue. J Clin Invest. 1967 Mar;46(3):287–298. doi: 10.1172/JCI105531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- KIVEL R. M., KEARNS D. H., LIEBOWITZ D. SIGNIFICANCE OF ANTIBODIES TO DIETARY PROTEINS IN THE SERUMS OF PATIENTS WITH NONTROPICAL SPRUE. N Engl J Med. 1964 Oct 8;271:769–772. doi: 10.1056/NEJM196410082711504. [DOI] [PubMed] [Google Scholar]
- Kumar P. J., O'Donoghue D. P., Stenson K., Dawson A. M. Reintroduction of gluten in adults and children with treated coeliac disease. Gut. 1979 Sep;20(9):743–749. doi: 10.1136/gut.20.9.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDEMANN B., SOLOMON A. K. Permeability of luminal surface of intestinal mucosal cells. J Gen Physiol. 1962 Mar;45:801–810. doi: 10.1085/jgp.45.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamabadusuriya S. P., Packer S., Harries J. T. Limitations of xylose tolerance test as a screening procedure in childhood coeliac disease. Arch Dis Child. 1975 Jan;50(1):34–39. doi: 10.1136/adc.50.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD W. C., BRANDBORG L. L., FLICK A. L., TRIER J. S., RUBIN C. E. STUDIES OF CELIAC SPRUE. IV. THE RESPONSE OF THE WHOLE LENGTH OF THE SMALL BOWEL TO A GLUTEN-FREE DIET. Gastroenterology. 1964 Dec;47:573–589. [PubMed] [Google Scholar]
- McNicholl B., Egan-Mitchell B., Fottrell P. F. Variability of gluten intolerance in treated childhood coeliac disease. Gut. 1979 Feb;20(2):126–132. doi: 10.1136/gut.20.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies I. S., Laker M. F., Pounder R., Bull J., Heyer S., Wheeler P. G., Creamer B. Abnormal intestinal permeability to sugars in villous atrophy. Lancet. 1979 Nov 24;2(8152):1107–1109. doi: 10.1016/s0140-6736(79)92507-8. [DOI] [PubMed] [Google Scholar]
- Menzies I. S. Quantitative estimation of sugars in blood and urine by paper chromatography using direct densitometry. J Chromatogr. 1973 Jun 27;81(1):109–127. doi: 10.1016/s0021-9673(01)82322-0. [DOI] [PubMed] [Google Scholar]
- Nasrallah S. M., Iber F. L. Mannitol absorption and metabolism in man. Am J Med Sci. 1969 Aug;258(2):80–88. doi: 10.1097/00000441-196908000-00003. [DOI] [PubMed] [Google Scholar]
- Packer S. M., Charlton V., Keeling J. W., Risdon R. A., Ogilvie D., Rowlatt R. J., Larcher V. F., Harries J. T. Gluten challenge in treated coeliac disease. Arch Dis Child. 1978 Jun;53(6):449–455. doi: 10.1136/adc.53.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña A. S., Truelove S. C., Whitehead R. Disaccharidase activity and jejunal morphology in coeliac disease. Q J Med. 1972 Oct;41(164):457–476. [PubMed] [Google Scholar]
- Pollock D. J., Nagle R. E., Jeejeebhoy K. N., Coghill N. F. The effect on jejunal mucosa of withdrawing and adding dietary gluten in cases of idiopathic steatorrhoea. Gut. 1970 Jul;11(7):567–575. doi: 10.1136/gut.11.7.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolles C. J., McNeish A. S. Standardised approach to gluten challenge in diagnosing childhood coeliac disease. Br Med J. 1976 May 29;1(6021):1309–1311. doi: 10.1136/bmj.1.6021.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiner M., Ballard J. Antigen-antibody reactions in jejunal mucosa in childhood coeliac disease after gluten challenge. Lancet. 1972 Jun 3;1(7762):1202–1205. doi: 10.1016/s0140-6736(72)90924-5. [DOI] [PubMed] [Google Scholar]
- Shiner M. Ultrastructural changes suggestive of immune reactions in the jejunal mucosa of coeliac children following gluten challenge. Gut. 1973 Jan;14(1):1–12. doi: 10.1136/gut.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. H., Wright E. M. Streaming potentials in the rat small intestine. J Physiol. 1966 Feb;182(3):591–602. doi: 10.1113/jphysiol.1966.sp007839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler P. G., Menzies I. S., Creamer B. Effect of hyperosmolar stimuli and coeliac disease on the permeability of the human gastrointestinal tract. Clin Sci Mol Med. 1978 May;54(5):495–501. doi: 10.1042/cs0540495. [DOI] [PubMed] [Google Scholar]


