Abstract
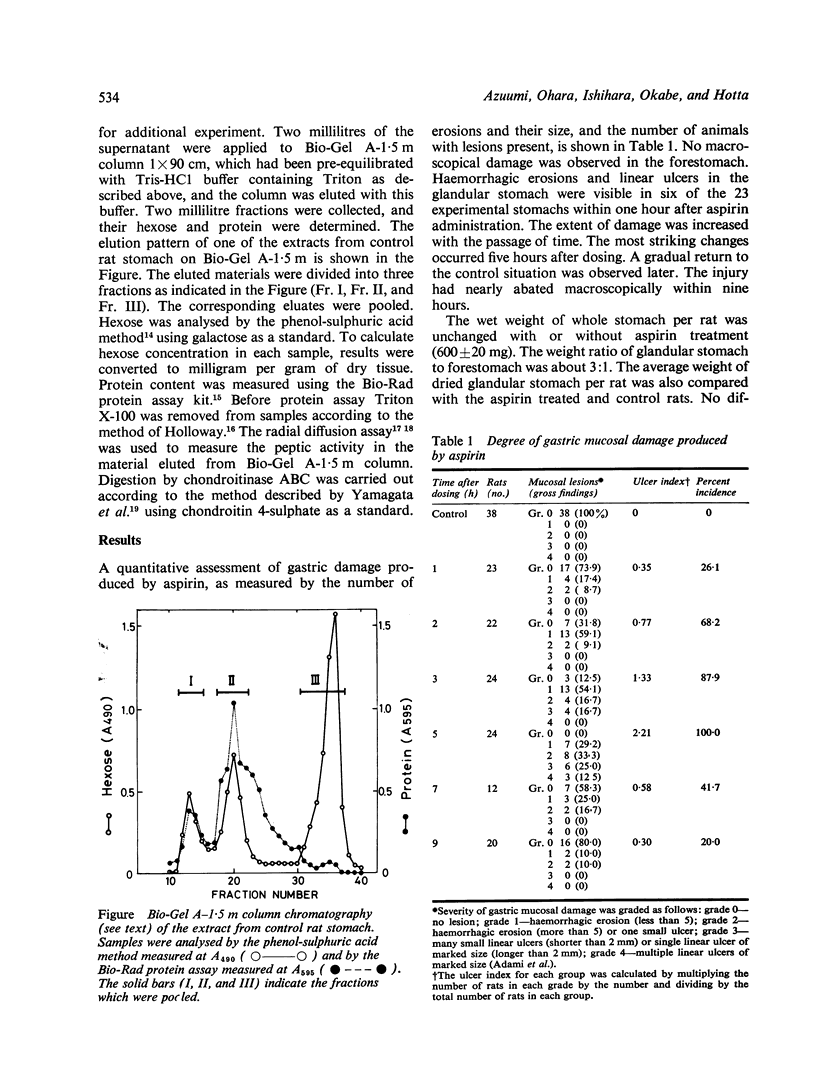
Quantitative changes of gastric mucosal glycoproteins with the gastric damage induced by acetylsalicylic acid (aspirin) in rat have been studied. Gastric injury was easily observed macroscopically within one hour after the oral administration of aspirin. The most striking changes occurred at five hours, and the injury was overcome within nine hours after dosing. The glycoproteins extracted from rat stomack with Tris buffer containing Triton X-100 were fractionated on Bio-Gel A-1.5 m column chromatography and divided into three fractions. The first peak, corresponding to gastric mucus macromolecular neutral and acidic glycoproteins with or without sulphate (Fr.I), was diminished after aspirin administration. A considerable alteration of Fr.I (49% of control) appeared at three hours, and a gradual return to the control value was observed subsequently. The changes in the amount of the glycoproteins were detected before the macroscopical changes of the mucosa. These results suggest that gastric ulceration induced by aspirin may be caused by a deficiency of gastric mucus macromolecular glycoproteins of gastric mucus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMI E., MARAZZI-UBERTI E., TURBA C. PHARMACOLOGICAL RESEARCH ON GEFARNATE, A NEW SYNTHETIC ISOPRENOID WITH AN ANTI-ULCER ACTION. Arch Int Pharmacodyn Ther. 1964 Jan 1;147:113–145. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Doleschel W., Auerswald W. Gel diffusion method for determination of pepsin. Clin Chim Acta. 1973 Nov 23;49(1):105–108. doi: 10.1016/0009-8981(73)90349-5. [DOI] [PubMed] [Google Scholar]
- Glass G. B. Current status of the "glandular mucoprotein" and "mucoproteose" fractions of the gastric mucin: a review of 15 years progress in this area. Ann N Y Acad Sci. 1967 Jan 26;140(2):804–834. doi: 10.1111/j.1749-6632.1967.tb51005.x. [DOI] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Ishida H., Hotta K., Tamechica Y., Okabe H. [An investigation of the measurement of gastric pepsin (author's transl)]. Nihon Shokakibyo Gakkai Zasshi. 1977 Jul;74(7):924–930. [PubMed] [Google Scholar]
- Kent P. W., Allen A. The biosynthesis of intestinal mucins. The effect of salicylate on glycoprotein biosynthesis by sheep colonic and human gastric mucosal tissues in vitro. Biochem J. 1968 Feb;106(3):645–658. doi: 10.1042/bj1060645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENGUY R., MASTERS Y. F. EFFECTS OF ASPIRIN ON GASTRIC MUCOUS SECRETION. Surg Gynecol Obstet. 1965 Jan;120:92–98. [PubMed] [Google Scholar]
- Mikuni-Takagaki Y., Hotta K. Characterization of peptic inhibitory activity associated with sulfated glycoprotein isolated from gastric mucosa. Biochim Biophys Acta. 1979 May 1;584(2):288–297. doi: 10.1016/0304-4165(79)90274-5. [DOI] [PubMed] [Google Scholar]
- Rainsford K. D. Electronmicroscopic observations on the effects of orally administered aspirin and aspirin-bicarbonate mixtures on the development of gastric mucosal damage in the rat. Gut. 1975 Jul;16(7):514–527. doi: 10.1136/gut.16.7.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter R. H. Aspirin and gastrointestinal bleeding. Am J Dig Dis. 1968 Jan;13(1):38–58. doi: 10.1007/BF02239210. [DOI] [PubMed] [Google Scholar]
- Schrager J., Oates M. D. A comparative study of the major glycoprotein isolated from normal and neoplastic gastric mucosa. Gut. 1973 Apr;14(4):324–329. doi: 10.1136/gut.14.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrager J., Oates M. D. The isolation and partial characterization of a glycoprotein isolated from human gastric aspirates and from extracts of gastric mucosae. Biochim Biophys Acta. 1974 Nov 4;372(1):183–195. doi: 10.1016/0304-4165(74)90086-5. [DOI] [PubMed] [Google Scholar]
- Schrager J. The composition and some structural features of the principal gastric glycoprotein. Digestion. 1969;2(2):73–89. doi: 10.1159/000196926. [DOI] [PubMed] [Google Scholar]
- Sheahan D. G., Jervis H. R. Comparative histochemistry of gastrointestinal mucosubstances. Am J Anat. 1976 Jun;146(2):103–131. doi: 10.1002/aja.1001460202. [DOI] [PubMed] [Google Scholar]
- Terho T. T., Hartiala K. Metabolic turnover of sulphated heteroglycans in the gastrointestinal tract, cartilage and skin of the rat. Biochim Biophys Acta. 1973 May 28;304(3):591–610. doi: 10.1016/0304-4165(73)90206-7. [DOI] [PubMed] [Google Scholar]
- Willems G. Cell renewal in the gastric mucosa. Digestion. 1972;6(1):46–63. doi: 10.1159/000197221. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]


